所有图片(1)
About This Item
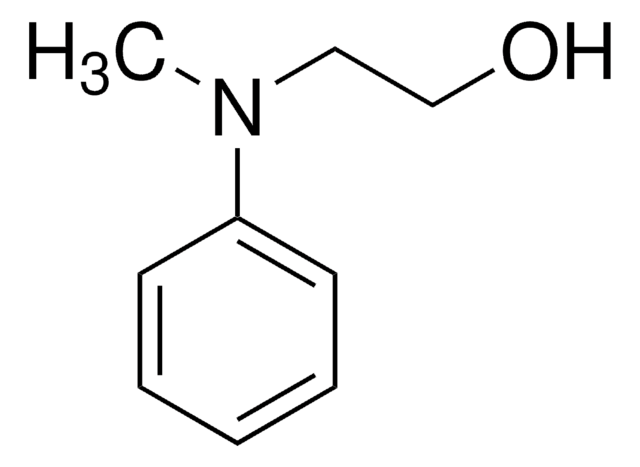
线性分子式:
C6H5NHCH2CH2OH
CAS号:
分子量:
137.18
Beilstein:
774672
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
蒸汽密度
>1 (vs air)
蒸汽压
<0.01 mmHg ( 20 °C)
方案
98%
表单
liquid
折射率
n20/D 1.578 (lit.)
沸点
278-282 °C/760 mmHg (lit.)
密度
1.094 g/mL at 25 °C (lit.)
SMILES字符串
OCCNc1ccccc1
InChI
1S/C8H11NO/c10-7-6-9-8-4-2-1-3-5-8/h1-5,9-10H,6-7H2
InChI key
MWGATWIBSKHFMR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
应用
N-(2-羟乙基)苯胺被用作人嗅觉UDP-葡糖醛酸糖基转移酶的底物。
警示用语:
Danger
危险分类
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - STOT RE 2 - STOT SE 1
靶器官
Blood, Blood,hematopoietic system
储存分类代码
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
闪点(°F)
235.4 °F - closed cup
闪点(°C)
113 °C - closed cup
个人防护装备
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Mercedes Amat et al.
Organic & biomolecular chemistry, 9(7), 2175-2184 (2011-02-08)
The double cyclocondensation of symmetric pyridyl bis(oxoacids) 2b and 3b with (R)-phenylglycinol stereoselectively gave access to bis-phenylglycinol-derived oxazolopyrrolidine 9 and oxazolopiperidone 10, respectively. Application of the stereocontrolled cyclocondensation reaction to phenyl bis-γ-oxoacid 4b provided 11, which was converted to the
M Amat et al.
Organic letters, 3(21), 3257-3260 (2001-10-12)
[reaction: see text]. The phenylglycinol-derived 2-pyridone 1 undergoes m-CPBA oxidation steroselectively leading to the chiral nonracemic unsaturated bicyclic hydroxylactam 2, from which the enantioselective synthesis of (3R,5R)-3,4,5-trihydroxypiperidine (16) and the formal synthesis of the azasugar epiisofagomine are described. The enantioselective
Mercedes Amat et al.
Natural product communications, 6(4), 515-526 (2011-05-13)
This review is focused on recent synthetic achievements and ongoing work in our laboratory using phenylglycinol-derived oxazolopiperidone lactams as starting materials for the enantioselective synthesis of piperidine-containing alkaloids: madangamines, 2,5-disubstituted decahydroquinoline and 1-substituted tetrahydroisoquinoline alkaloids, the indole alkaloids 20S- and
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(28), 7724-7732 (2011-06-15)
Phenylglycinol-derived, unsaturated oxazolopiperidone lactams are extremely useful building blocks that undergo stereoselective conjugate addition reactions with organocuprates, enolates, and sulfur-stabilized anions, allowing the stereocontrolled introduction of substituents at the piperidine 4-position. The factors governing the exo- or endo-facial selectivity are
Mercedes Amat et al.
The Journal of organic chemistry, 71(10), 3804-3815 (2006-05-06)
The stereochemical outcome of the alkylation of a variety of phenylglycinol-derived oxazolopiperidone lactams is studied. The influence of the configuration of the C-8a stereocenter and the effect of the substituents at the C-8 and C-8a positions on the stereoselectivity of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门