推荐产品
等級
technical grade
品質等級
化驗
94%
形狀
liquid
反應適用性
reaction type: C-C Bond Formation
折射率
n20/D 1.396 (lit.)
bp
102-103 °C/720 mmHg (lit.)
密度
0.897 g/mL at 25 °C (lit.)
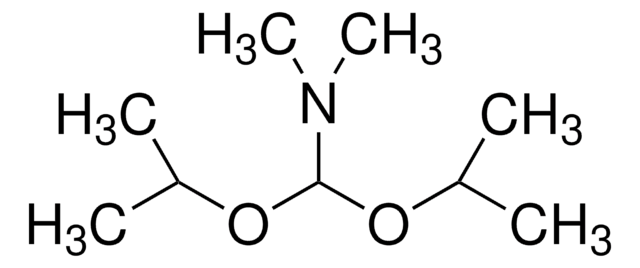
SMILES 字串
COC(OC)N(C)C
InChI
1S/C5H13NO2/c1-6(2)5(7-3)8-4/h5H,1-4H3
InChI 密鑰
ZSXGLVDWWRXATF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
N,N-二甲基甲酰胺二甲基缩醛(DMF-DMA)可用作:
- 有机催化剂,以通过芳乙烯与缺电子炔烃反应合成1,2-二取代-3,4-二氢萘。
- 基本构成要素,以通过亚甲基和氨基的甲酰化制备诸如烯胺和脒等杂环化合物。
- 一种试剂,以通过与氯代乙酰胺反应合成1,4-二芳基-哌嗪-2,5-二酮。
- 一种试剂,在Me3SiCl存在下,与烯胺和炔属烃一起通过三组分偶联反应制备2,3,4,5-四取代吡啶衍生物。
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
42.8 °F
閃點(°C)
6 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
A highly efficient cycloaddition of vinylarenes with electron-deficient alkynes affording 1, 2-disubstituted-3, 4-dihydronaphthalenes catalysed by N, N-dimethylformamide dimethyl acetal
Jiang J-L, et al.
Organic & Biomolecular Chemistry, 5(12), 1854-1857 (2007)
Synthesis of 1, 4-Diaryl-piperazine-2, 5-diones: New Behavior of N, N-Dimethylformamide Dimethyl Acetal (DMFDMA)
Abu-Shanab FA, et al.
Synthetic Communications, 38(3), 376-382 (2008)
Dimethylformamide dimethyl acetal as a building block in heterocyclic synthesis
Abu-Shanab FA, et al.
Journal of Heterocyclic Chemistry, 46(5), 801-827 (2009)
Wim Schepens et al.
Organic letters, 8(19), 4247-4250 (2006-09-08)
A novel series of analogues of calcitriol (1) is developed featuring a spirocyclic central core resulting from C18/C21-connection and C15/C16-deletion (2a, 2b). The synthesis of the key intermediate involves an Eschenmoser rearrangement of an enantiomerically pure bromo-substituted cyclohexenol.
M F Grubb et al.
Journal of chromatography, 469, 191-196 (1989-05-19)
Six amino acids containing either an N-methyl or a cyclic secondary amine were converted to volatile derivatives by reaction with dimethylformamide dimethyl acetal. The amine functionalities were formylated by way of an amide acetal intermediate while the carboxylic acid groups
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门