144185
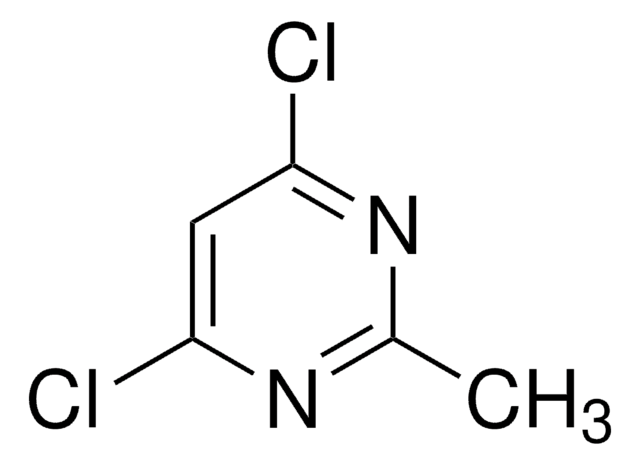
2,4-Dichloro-6-methylpyrimidine
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C5H4Cl2N2
Numéro CAS:
Poids moléculaire :
163.00
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
98%
Forme
solid
Point d'ébullition
219 °C (lit.)
Pf
44-47 °C (lit.)
Groupe fonctionnel
chloro
Chaîne SMILES
Cc1cc(Cl)nc(Cl)n1
InChI
1S/C5H4Cl2N2/c1-3-2-4(6)9-5(7)8-3/h2H,1H3
Clé InChI
BTLKROSJMNFSQZ-UHFFFAOYSA-N
Description générale
2,4-Dichloro-6-methylpyrimidine undergoes double cross-coupling reaction with 2-(tributylstannyl)pyridine, followed by aldol condensation to yield 4-arylvinyl-2,6-di(pyridin-2-yl)pyrimidines. It reacts with 1H,1H,2H,2H-perfluorodecanethiol during fluorous synthesis of disubstituted pyrimidines.
Application
2,4-Dichloro-6-methylpyrimidine was used in the synthesis of (2-chloro-6-methyl-pyrimidin-4-yl)-(2,3-dihydro-benzothiazol-6-yl)-amine.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
235.4 °F - closed cup
Point d'éclair (°C)
113 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Caroline Hadad et al.
The Journal of organic chemistry, 76(10), 3837-3845 (2011-04-06)
A series of 4-arylvinyl-2,6-di(pyridin-2-yl)pyrimidines have been efficiently prepared by a double cross-coupling reaction between 2,4-dichloro-6-methylpyrimidine and 2-(tributylstannyl)pyridine, followed by aldol condensation with the appropriate aromatic aldehyde substituted with electron-donating, electron-withdrawing, dendritic, or water-soluble groups. The effect of different protic and
Eliud Hernández et al.
Puerto Rico health sciences journal, 29(4), 348-356 (2011-01-26)
Rho family GTPases are molecular switches that control signaling pathways regulating a myriad of cellular functions. Rac1, a Rho family member, plays a critical role in several aspects of tumorigenesis, cancer progression, invasion, and metastasis. Rac proteins are not mutated
Wei Zhang
Organic letters, 5(7), 1011-1013 (2003-03-28)
[reaction: see text] The fluorous synthesis of disubstituted pyrimidines is carried out by attaching 2,4-dichloro-6-methylpyrimidine with 1H,1H,2H,2H-perfluorodecanethiol. The tagged substrate is substituted with 3-(trifluoromethyl)pyrazole followed by thioether oxidation and tag displacement with amines or thiols. The fluorous chain serves as
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![[1,1′-bis(diphénylphosphino)ferrocène]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphénylphosphino)ferrocène]dichloropalladium(II), complexe avec le dichlorométhane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)