Alle Fotos(1)
Wichtige Dokumente
493937
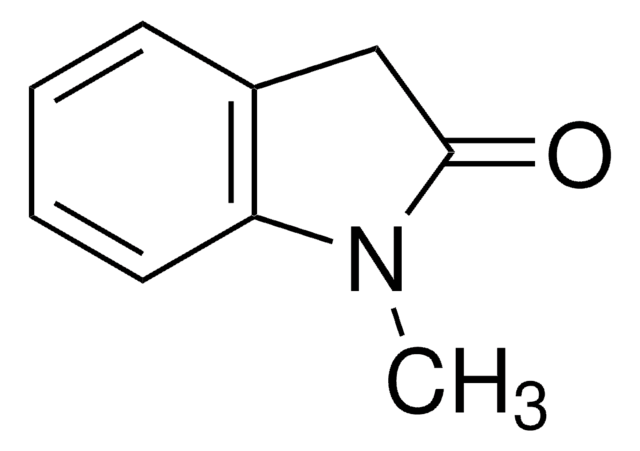
3-Methoxyindol
96%
Synonym(e):
3-Methyl-2-oxindol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C9H9NO
CAS-Nummer:
Molekulargewicht:
147.17
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
96%
Form
solid
mp (Schmelzpunkt)
117-121 °C (lit.)
SMILES String
CC1C(=O)Nc2ccccc12
InChI
1S/C9H9NO/c1-6-7-4-2-3-5-8(7)10-9(6)11/h2-6H,1H3,(H,10,11)
InChIKey
BBZCPUCZKLTAJQ-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products.
Anwendung
3-Methyl-2-oxindole may be used in the preparation of 3-hydroxy-3-methyl-2-oxindole.
- Reactant for enantioselective α-amination reactions
- Reactant for aldol reaction with glyoxal derivatives
- Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes
- Reactant for O-acetylation reactions
- Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Facile and Efficient Enantioselective Hydroxyamination Reaction: Synthesis of 3-Hydroxyamino-2-Oxindoles Using Nitrosoarenes.
Shen K, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 123(20), 4780-4784 (2011)
Ying Jin et al.
Chirality, 26(12), 801-805 (2014-07-22)
A series of cinchona alkaloid derivatives were used to catalyze the asymmetric anti-Mannich-type reaction of 3-methyl-2-oxindole with N-tosyl aryl aldimines. The resulting anti-3,3-disubstituted 2-oxindole products were obtained in good yields (up to 92%) with high diastereo- and enantioselectivities (anti/syn up
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-carbinol, and 3-methylindole in goats.
M J Potchoiba et al.
American journal of veterinary research, 43(8), 1418-1423 (1982-08-01)
Zhengyin Yan et al.
Analytical chemistry, 80(16), 6410-6422 (2008-07-23)
Constant neutral loss (CNL) and precursor ion (PI) scan have been widely used for the in vitro screening of glutathione conjugates derived from reactive metabolites, but these two methods are only applicable to triple quadrupole or hybrid triple quadrupole mass
Takuma Sakurai et al.
Microorganisms, 7(9) (2019-09-14)
Recent studies have shown that metabolites produced by microbes can be considered as mediators of host-microbial interactions. In this study, we examined the production of tryptophan metabolites by Bifidobacterium strains found in the gastrointestinal tracts of humans and other animals.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.