Alle Fotos(3)
Wichtige Dokumente
O9808
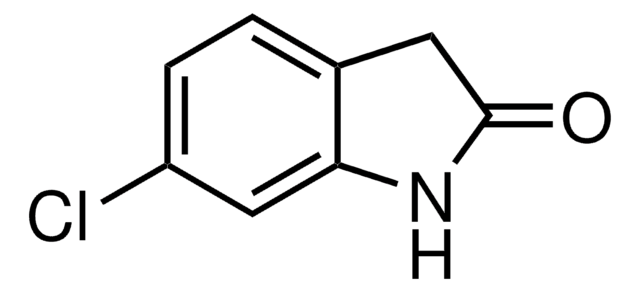
2-Indolinon
97%
Synonym(e):
2-Oxindol, Oxindol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C8H7NO
CAS-Nummer:
Molekulargewicht:
133.15
Beilstein:
114692
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
crystals
bp
227 °C/73 mmHg (lit.)
mp (Schmelzpunkt)
123-128 °C (lit.)
SMILES String
O=C1Cc2ccccc2N1
InChI
1S/C8H7NO/c10-8-5-6-3-1-2-4-7(6)9-8/h1-4H,5H2,(H,9,10)
InChIKey
JYGFTBXVXVMTGB-UHFFFAOYSA-N
Angaben zum Gen
human ... PGR(5241)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
P B Bergqvist et al.
Pharmacology & toxicology, 85(3), 138-143 (1999-10-16)
It has previously been shown that the neurodepressant L-tryptophan metabolite oxindole is increased in the blood and brain of rats with fulminant hepatic failure and in the blood of cirrhotic patients affected by chronic hepatic encephalopathy. In the present investigation
R Carpenedo et al.
Journal of neurochemistry, 70(5), 1998-2003 (1998-05-08)
Rats treated with oxindole (10-100 mg/kg i.p.), a putative tryptophan metabolite, showed decreased spontaneous locomotor activity, loss of the righting reflex, hypotension, and reversible coma. Brain oxindole levels were 0.05 +/- 0.01 nmol/g in controls and increased to 8.1 +/-
Oxindole in pathogenesis of hepatic encephalopathy.
F Moroni et al.
Lancet (London, England), 351(9119), 1861-1861 (1998-07-04)
Tao Zhang et al.
Chemical communications (Cambridge, England), 49(16), 1636-1638 (2013-01-24)
An enantioselective direct α-alkylation of 2-oxindoles with Michler's hydrol via an S(N)1-type pathway in the non-covalent activation mode using the bis-cinchona alkaloid and Brønsted acid as a co-catalyst was developed and good to high yields and enantioselectivities were obtained.
Zhigang Yang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(22), 6632-6637 (2010-04-22)
A highly enantioselective alpha-amination of 3-substituted oxindoles with azodicarboxylates catalyzed by a chiral Sc(OTf)(3)/N,N'-dioxide complex (Tf: triflate) has been developed and affords the corresponding 3-amino-2-oxindole derivatives in high yields (up to 98%) with excellent enantioselectivities (up to 99% ee). The
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.