129445
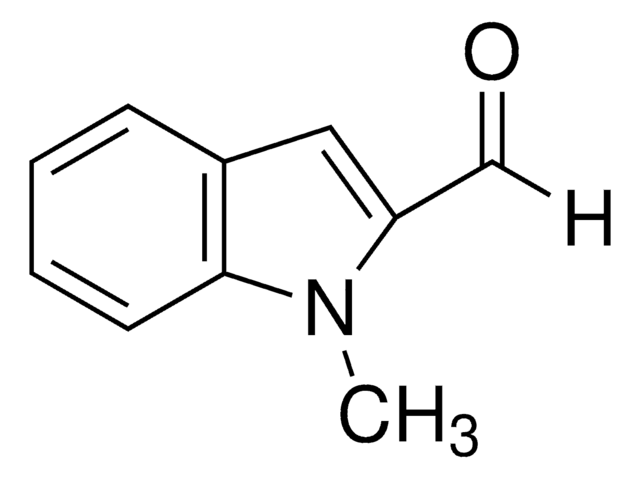
Indol-3-carboxaldehyd
97%
Synonym(e):
β-Indolylaldehyde, 3-Formylindole, 3-Indolylformaldehyde, Indole-3-carbaldehyde, NSC 10118
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C9H7NO
CAS-Nummer:
Molekulargewicht:
145.16
Beilstein:
114117
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
solid
mp (Schmelzpunkt)
193-198 °C (lit.)
Funktionelle Gruppe
aldehyde
SMILES String
O=Cc1c[nH]c2ccccc12
InChI
1S/C9H7NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-6,10H
InChIKey
OLNJUISKUQQNIM-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Indole-3-carboxaldehyde can undergo Schiff bases condensation to form multifunctional silica nano-vehicles and magnetic nanoparticles.
Anwendung
Starting material for the synthesis of higher order indoles including isoindolo[2,1-a]indoles, aplysinopsins, and 4-substituted-tetrahydrobenz[cd]indoles.
Reactant for preparation of:
- Analgesic agents
- Hypoglycemic agents
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Antibacterial and antifungal agents
- Antiamoebic and cytotoxic agents
- Inhibitors of the Dengue virus protease with antiviral activity in cell-culture
- Curcumin analogues as possible anti-proliferative & anti-inflammatory agents
- Inhibitors of Bcl-2 family proteins
- Inhibitors of the C-terminal domain of RNA Polymerase II as antitumor agents
- Inhibitors of TNF-α and IL-6 with anti-tubercular activity
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Indian J. Chem. B, 33, 4-4 (1994)
Heterocycles, 38, 1479-1479 (1994)
Mariana Budovská et al.
Bioorganic & medicinal chemistry, 21(21), 6623-6633 (2013-09-10)
An effective synthesis of analogs of the indole phytoalexin cyclobrassinin with NR1R2 group instead of SCH3 was developed starting from indole-3-carboxaldehyde. The target compounds were prepared by spirocyclization of 1-Boc-thioureas with the formation of isolable spiroindoline intermediates, followed by the
Qiu-Yun Chen et al.
Colloids and surfaces. B, Biointerfaces, 114, 158-163 (2013-11-05)
Multifunctional silica nano-vehicles (SiO2@indol-IL) and magnetic nanoparticles (Fe3O4@indol-IL) were constructed through the Schiff bases condensation of indole-3-carboxaldehyde and 4-acetyl-N-allyl pyridinium chloride (ILs) with the amine groups of silica and magnetic nanoparticles. SiO2@indol-IL can inhibit the proliferation of HepG-2 cells in
S Muthu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 106, 299-309 (2013-02-19)
Indole-3-Aldehyde is a new organic non-linear material having good second harmonic generation. The optimized molecular geometry, harmonic vibrational frequencies, infrared intensities of Indole-3-Aldehyde (I3A, C9H7NO) in the ground state were carried out by using density functional theory (B3LYP) method with
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.