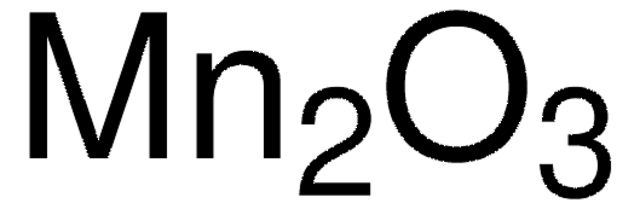377473
Mangan(II,III)-oxid
97%
Synonym(e):
MnO.Mn2O3
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
Mn3O4
CAS-Nummer:
Molekulargewicht:
228.81
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352303
PubChem Substanz-ID:
NACRES:
NA.23
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
powder
Dichte
4.8 g/mL at 25 °C (lit.)
Anwendung(en)
battery manufacturing
SMILES String
O=[Mn]O[Mn]O[Mn]=O
InChI
1S/3Mn.4O
InChIKey
GVNFAUMGUISVJW-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Manganese(II,III) oxide is a transition metal oxide that is formed by annealing manganese oxide in the air above 1000°C. It can be used for a variety of applications such as catalysis, electrochromic devices, and other energy storage applications.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Repr. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
nwg
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Chemistry of elements (2012)
Lucas K Wagner
The Journal of chemical physics, 138(9), 094106-094106 (2013-03-15)
Very accurate wave functions are calculated for small transition metal oxide molecules. These wave functions are decomposed using reduced density matrices to study the underlying correlation of electrons. The correlation is primarily of left-right type between the transition metals and
Andres M Cardenas-Valencia et al.
Rapid communications in mass spectrometry : RCM, 27(5), 635-642 (2013-02-16)
In situ analytical techniques that require the storage and delivery of reagents (e.g., acidic or basic solutions) have inherent durability limitations. The reagentless electrolytic technique for pH modification presented here was developed primarily to ease and to extend the longevity
Y Zhang et al.
Journal of hazardous materials, 248-249, 81-88 (2013-01-23)
Several MCM-41 materials were synthesized at different conditions by hydrothermal procedure using cheap and easily available industrial water glass as silica source. Fe doped manganese-based oxide/MCM-41 sorbents were prepared by a sol-gel method. The effects of loadings of metal oxide
Nancy Birkner et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(22), 8801-8806 (2013-05-15)
Previous measurements show that calcium manganese oxide nanoparticles are better water oxidation catalysts than binary manganese oxides (Mn3O4, Mn2O3, and MnO2). The probable reasons for such enhancement involve a combination of factors: The calcium manganese oxide materials have a layered
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.