15404
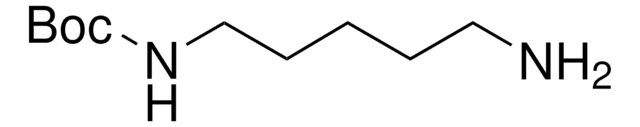
N-Boc-1,4-butandiamin
≥97.0% (GC/NT)
Synonym(e):
N-(4-Aminobutyl)-carbamidsäure-tert.-butylester, N-Boc-1,4-diamino-butan
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥97.0% (GC/NT)
Eignung der Reaktion
reagent type: cross-linking reagent
Brechungsindex
n20/D 1.460
Dichte
0.984 g/mL at 20 °C (lit.)
Funktionelle Gruppe
Boc
amine
SMILES String
NCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H20N2O2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7,10H2,1-3H3,(H,11,12)
InChIKey
ZFQWJXFJJZUVPI-UHFFFAOYSA-N
Anwendung
- Carboxy-Silane Coated Iron Oxide Nanoparticles: Details the application of N-Boc-1,4-butanediamine in modifying iron oxide nanoparticles for imaging and drug delivery (D Stanicki, S Boutry, S Laurent, et al., 2014). Access the article.
Sonstige Hinweise
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
228.2 °F - closed cup
Flammpunkt (°C)
109.0 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.