Alle Fotos(1)
Wichtige Dokumente
115312
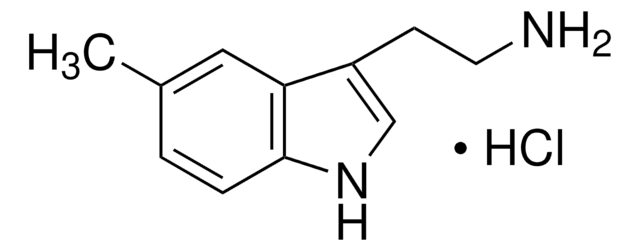
N-ω-Methyltryptamin
99%
Synonym(e):
3-(2-Methylaminoethyl)-indol, 3-(2-[Methylamino]-ethyl)-indol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C11H14N2
CAS-Nummer:
Molekulargewicht:
174.24
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Form:
solid
Assay:
99%
Empfohlene Produkte
Qualitätsniveau
Assay
99%
Form
solid
mp (Schmelzpunkt)
87-89 °C (lit.)
Funktionelle Gruppe
amine
SMILES String
CNCCc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-12-7-6-9-8-13-11-5-3-2-4-10(9)11/h2-5,8,12-13H,6-7H2,1H3
InChIKey
NCIKQJBVUNUXLW-UHFFFAOYSA-N
Anwendung
N-ω-Methyltryptamine was used in the preparation of N-acetyl-α−methyltryptamine.
N-ω-methyltryptamine was used in the biosynthesis of dolichantoside using U. tomentosa protein extracts.
Reactant for preparation of:
- Manzamine analogues for the control of neuroinflammation and cerebral infections
- Serotonin 4 receptors (5-HT4) receptor agonists
- A sulful-containing indole alkaloid, glypetelotine
- Selective inhibitors of cyclin dependent kinase (CDK4)
- Antagonist of the human tachykinin NK-2 receptor
- Inhibitors of the tyrosine-specific protein kinase pp60c-src SH2 Domain
Verpackung
Bottomless glass bottle. Contents are inside inserted fused cone.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
T Takahashi et al.
The International journal of applied radiation and isotopes, 36(12), 965-969 (1985-12-01)
Five indolealkylamines (N,N-dimethyltryptamine, N-methyltryptamine, bufotenine, O-methylbufotenine, N,N,N-trimethyltryptamine iodide) were labeled with 11C by use of 11CH3I. The labeled compounds were synthesized with a radiochemical yield of 2-50% (based on trapped 11CH3I) in 20-35 min with radiochemical purities of more than
W J VandenHeuvel et al.
Journal of chromatographic science, 21(3), 119-124 (1983-03-01)
This report focuses on recent applications of capillary column GLC to the analysis of drugs and metabolites, other xenobiotics, natural products, and environmental contaminants in samples of biological origin. The increasing use of selected ion monitoring, combined with stable isotope
R N Cory et al.
The Journal of pharmacology and experimental therapeutics, 236(1), 48-54 (1986-01-01)
The contractile response of the isolated rabbit aorta elicited by 5-hydroxytryptamine (5-HT) and five partial agonists acting on the 5-HT2 receptor were separated into a phasic and a tonic response by altering the [Ca++] in the buffer. A kinetic analysis
R W Walker et al.
Journal of chromatography, 289, 223-229 (1984-04-27)
A capillary column gas-liquid chromatography selected ion monitoring-based method was developed for the measurement of [13C,15N]N-methyltryptamine ( NMT ) in human urine. The method was employed to establish the extent of conversion of [13C,15N]tryptamine to the correspondingly labeled NMT in
T J Williams et al.
European journal of pharmacology, 245(3), 197-201 (1993-05-15)
The binding of [3H]5-hydroxytryptamine (5-HT) to rat enteric membranes was inhibited by the inclusion of 5-HT 2-methyl-5-HT, 5-hydroxytryptophan, N,N,N-triethyltryptamine and 2-Br-N,N-diethyltryptamine in the incubation buffer. In contrast, tryptamine, 5-methoxytryptamine and 2-methyl-N,N-diethyltryptamine enhanced binding. Ascorbate and dithiothreitol facilitated and reduced binding
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indol 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)