All Photos(1)
About This Item
Empirical Formula (Hill Notation):
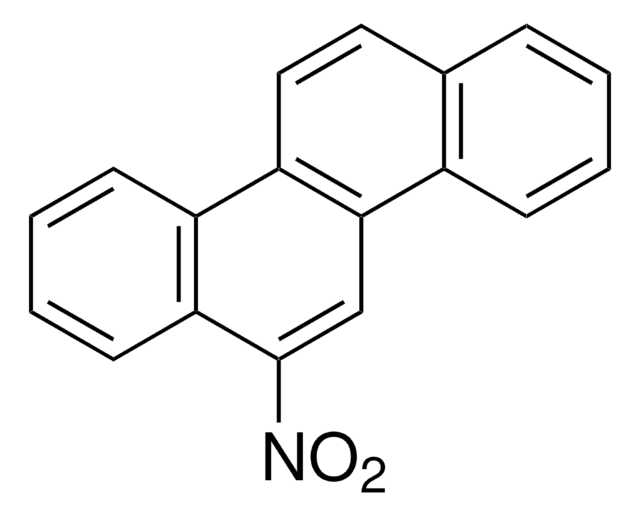
C14H9NO2
CAS Number:
Molecular Weight:
223.23
Beilstein:
1877509
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
93%
form
powder
mp
141-144 °C (lit.)
SMILES string
[O-][N+](=O)c1c2ccccc2cc3ccccc13
InChI
1S/C14H9NO2/c16-15(17)14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-9H
InChI key
LSIKFJXEYJIZNB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karla I Garfias-Gonzalez et al.
Molecules (Basel, Switzerland), 20(5), 8548-8559 (2015-05-20)
Two new classes of dendrimers bearing 8 and 32 fluorene donor groups have been synthesized. The first and second generations of these porphyrin-PAMAM-fluorene dendrimers were characterized by 1H-NMR, 13C-NMR, FTIR, UV-vis spectroscopy, elemental analyses and MALDI-TOF mass spectrometry. The UV-vis
Kiyoshi Fukuhara et al.
Bioorganic & medicinal chemistry, 15(11), 3869-3873 (2007-04-03)
Anthraquinones are typical photosensitizers used in photodynamic therapy (PDT). However, systemic toxicity is a major problem for anthraquinones due to their ability not only to bind DNA but also to cause oxidative stress even without photoirradiation. To avoid such disadvantages
P P Fu et al.
Carcinogenesis, 6(5), 753-757 (1985-05-01)
Aerobic metabolism of 9-nitroanthracene by uninduced rat liver microsomes produced four metabolites identified as trans-1,2- and 3,4-dihydrodiols, 1,2,3,4-tetrahydrotetrol of 9-nitroanthracene, and anthraquinone. Further metabolism of the predominant metabolite, 9-nitroanthracene trans-3,4-dihyrodiol, yielded a 1,2,3,4-tetrahydrotetrol with a trans-cis-trans configuration, indicating that a
Andrea Alparone et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 89, 129-136 (2012-01-20)
Structure, IR and Raman spectra of 1-, 2- and 9-nitroanthracene isomers (1-NA, 2-NA and 9-NA) were calculated and analyzed through density functional theory computations using the B3LYP functional with the 6-311+G** basis set. Steric and π-conjugative effects determine the characteristic
9-Nitroanthracene.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 33, 179-185 (1984-04-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service