All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H8N2O4
CAS Number:
Molecular Weight:
292.25
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
solubility
DMSO: soluble 2 mg/mL, clear, yellow to orange
functional group
nitro
SMILES string
[O-][N+](=O)c1cc([N+]([O-])=O)c2ccc3cccc4ccc1c2c34
InChI
1S/C16H8N2O4/c19-17(20)13-8-14(18(21)22)12-7-5-10-3-1-2-9-4-6-11(13)16(12)15(9)10/h1-8H
InChI key
KTNUVDBUEAQUON-UHFFFAOYSA-N
Related Categories
General description
The carcinogenecity of 1,3-dinitropyrene was studied in newborn female rats.
Application
1,3-Dinitropyrene has been used in:
- modification of the umu-assay (ISO 13829) to assess the cytotoxic potential of toxins
- in vitro synthesis of 1,N6-etheno-2′-deoxyadenosine and 1,N2-etheno-2′-deoxyguanosine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A K Hajos et al.
Journal of biochemical toxicology, 6(4), 277-282 (1991-01-01)
The effect of highly purified rat liver cytosolic NAD(P)H-quinone oxidoreductase [EC 1.6.99.2] on the mutagenicity of 1,3- 1,6- and 1,8-dinitropyrene (DNP) was studied in the Ames Salmonella typhimurium mutagenicity assay. NAD(P)H-quinone oxidoreductase over the range of 0.02-0.8 micrograms/plate (38-1500) units
C A Norman et al.
Carcinogenesis, 10(7), 1323-1327 (1989-07-01)
Formation of DNA adducts, following treatment of primary rabbit tracheal epithelial cells (RTEC) with 1,8-dinitropyrene (1,8-DNP) and its partially reduced derivative, 1-nitro-8-nitrosopyrene (1,8-NONO2), was examined using the 32P-post-labelling technique. Treatment of aerobic cells with 1,8-DNP or 1,8-NONO2 produced qualitatively similar
K Imaida et al.
Carcinogenesis, 16(12), 3027-3030 (1995-12-01)
The carcinogenicities of 1-nitropyrene (1-NP), 4-nitropyrene (4-NP), 1,3-dinitropyrene (1,3-DNP), 1,6-dinitropyrene (1,6-DNP), 1,8-dinitropyrene (1,8-DNP), 3-hydroxy-1-nitropyrene (3-OH-1-NP) and a mixture of 6- and 8-hydroxy-1-nitropyrene (6/8-OH-1-NP) were investigated in newborn female rats. Newborn female CD rats were treated s.c. eight times at weekly
G W Winston et al.
Mutation research, 279(4), 289-298 (1992-06-16)
The effects of chronic ethanol feeding of rats on the ability of liver fractions to modulate the bacterial mutagenicity of three dinitropyrene isomers (1,3-, 1,6- and 1,8-DNP), which require bacterial enzymes but not an exogenous enzyme source for activation, were
T Shimada et al.
Cancer research, 50(7), 2036-2043 (1990-04-01)
NADPH-fortified human liver microsomes were examined with regard to ability to detoxicate several chemicals that do not require enzymatic oxidation to elicit a genotoxic response in a Salmonella typhimurium TA1535/pSK1002 system where umu response is used as an indicator of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service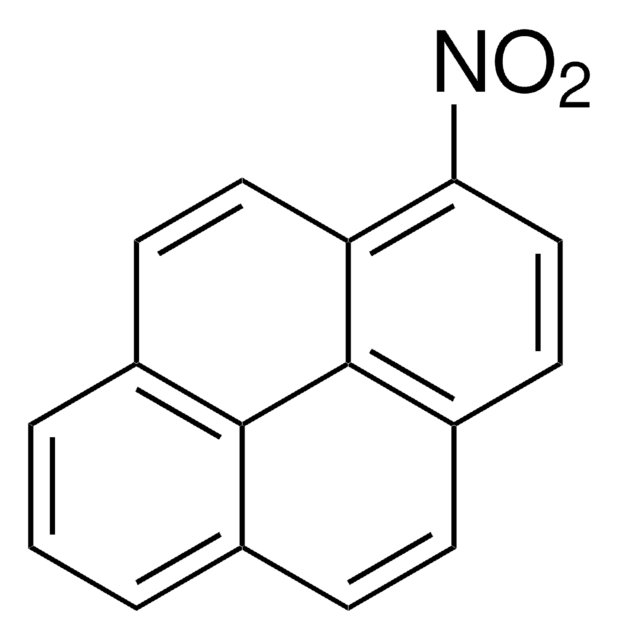






![1-(6-Methoxybenzo[d] thiazol-2-yl)hydrazine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/210/241/0c5be390-b73a-436d-82c7-c51156617e66/640/0c5be390-b73a-436d-82c7-c51156617e66.png)