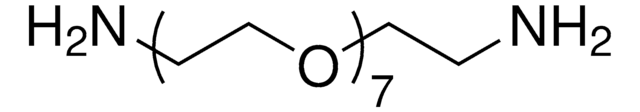14502
Poly(ethylene glycol) bis(amine)
Mw 3,000, carboxyl reactive, amine
Synonym(s):
Polyethylene glycol, O,O′-Bis(2-aminoethyl)polyethylene glycol, Diaminopolyethylene glycol, PEG-diamine, Polyoxyethylene bis(amine)
About This Item
Recommended Products
product name
Poly(ethylene glycol) bis(amine), Mw 3,000
mol wt
Mw 3,000
Quality Level
reaction suitability
reagent type: cross-linking reagent
reactivity: carboxyl reactive
Ω-end
amine
α-end
amine
polymer architecture
shape: linear
functionality: homobifunctional
InChI
1S/C6H16N2O2/c7-1-3-9-5-6-10-4-2-8/h1-8H2
InChI key
IWBOPFCKHIJFMS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Progress in biotechnology fields such as tissue engineering and drug delivery is accompanied by an increasing demand for diverse functional biomaterials. One class of biomaterials that has been the subject of intense research interest is hydrogels, because they closely mimic the natural environment of cells, both chemically and physically and therefore can be used as support to grow cells. This article specifically discusses poly(ethylene glycol) (PEG) hydrogels, which are good for biological applications because they do not generally elicit an immune response. PEGs offer a readily available, easy to modify polymer for widespread use in hydrogel fabrication, including 2D and 3D scaffold for tissue culture. The degradable linkages also enable a variety of applications for release of therapeutic agents.
Designing biomaterial scaffolds mimicking complex living tissue structures is crucial for tissue engineering and regenerative medicine advancements.
Designing biomaterial scaffolds mimicking complex living tissue structures is crucial for tissue engineering and regenerative medicine advancements.
Designing biomaterial scaffolds mimicking complex living tissue structures is crucial for tissue engineering and regenerative medicine advancements.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service