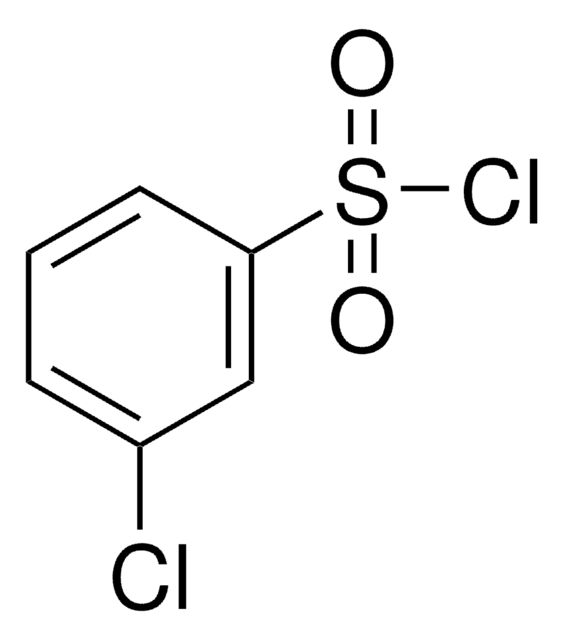
108669
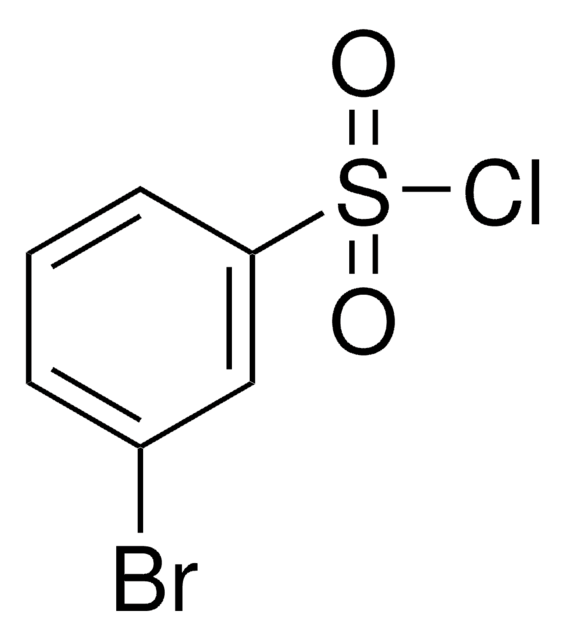
4-Bromobenzenesulfonyl chloride
98%
Synonym(s):
p-Bromobenzenesulfonyl chloride, Brosyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H4SO2Cl
CAS Number:
Molecular Weight:
255.52
Beilstein:
743518
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
153 °C/15 mmHg (lit.)
mp
73-75 °C (lit.)
functional group
bromo
SMILES string
ClS(=O)(=O)c1ccc(Br)cc1
InChI
1S/C6H4BrClO2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H
InChI key
KMMHZIBWCXYAAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Bromobenzenesulfonyl chloride was used as activating agent in the synthesis of oligodeoxyribo- and oligoribo- nucleotides in solution. It was used in the synthesis of 4-(N-allylsulfamoyl)phenylboronic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiaobao Li et al.
Analytical biochemistry, 372(2), 227-236 (2007-10-20)
Two new types of boronate affinity solid phases were synthesized and characterized. The materials were prepared by silylation of porous silica gel with monochlorosilane derivatives containing synthetic sulfonyl- and sulfonamide-substituted phenylboronic acids. The new solid phases were evaluated for boronate
Phosphotriester approach to the synthesis of oligonucleotides: a reappraisal.
Reese CB and Pei-Zhuo Z.
Journal of the Chemical Society. Perkin Transactions 1, 1(19), 2291-2301 (1993)
Tsurng-Juhn Huang et al.
European journal of medicinal chemistry, 90, 428-435 (2014-12-03)
Hepatitis B virus (HBV) is a causative reagent that frequently causes progressive liver diseases, leading to the development of acute, chronic hepatitis, cirrhosis, and eventually hepatocellular carcinoma (HCC). Despite several antiviral drugs including interferon-α and nucleotide derivatives are approved for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service