108472
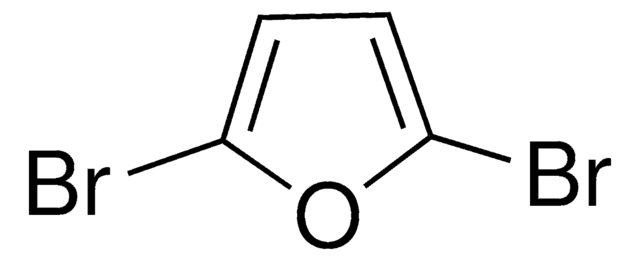
2,5-Dibromothiophene
95%
Synonym(s):
2,5-dibromo-thiophene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H2Br2S
CAS Number:
Molecular Weight:
241.93
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.629 (lit.)
bp
211 °C (lit.)
mp
−6 °C (lit.)
density
2.147 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Brc1ccc(Br)s1
InChI
1S/C4H2Br2S/c5-3-1-2-4(6)7-3/h1-2H
InChI key
KBVDUUXRXJTAJC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,5-Dibromothiophene polymerizes by debromination with magnesium catalyzed by nickel compounds to form poly(2,5- thienylene) .
Application
2,5-Dibromothiophene was used as starting reagent for the synthesis of α,α′-didecylquater-, -quinque- and -sexi-thiophenes. It was used in the preparation of soluble α,ω-diformyl-a-oligothiophenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of thermostable and electric-conducting poly (2, 5-thienylene).
Yamamoto T, et al.
Journal of Polymer Science - Part C: Polymer Letters, 18(1), 9-12 (1980)
Synthesis and thermal behaviour of α, α'-didecyloligothiophenes.
Ponomarenko S and Kirchmeyer S.
Journal of Materials Chemistry, 13(2), 197-202 (2003)
Synthesis and characterization of 3−hexyl multi−substituted α, ω−diformyl−α−oligothiophenes (n= 3, 6, 8).
Olinga T, et al.
Macromolecular Chemistry and Physics, 198(4), 1091-1107 (1997)
Minami Kato et al.
ChemSusChem, 13(9), 2379-2385 (2020-02-11)
Many types of batteries have been proposed as next-generation energy-storage systems. One candidate is a rocking-chair-type "molecular ion battery" in which a molecular ion, instead of Li+ , works as a charge carrier. Previously, we reported a viologen-type derivative as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service