247154
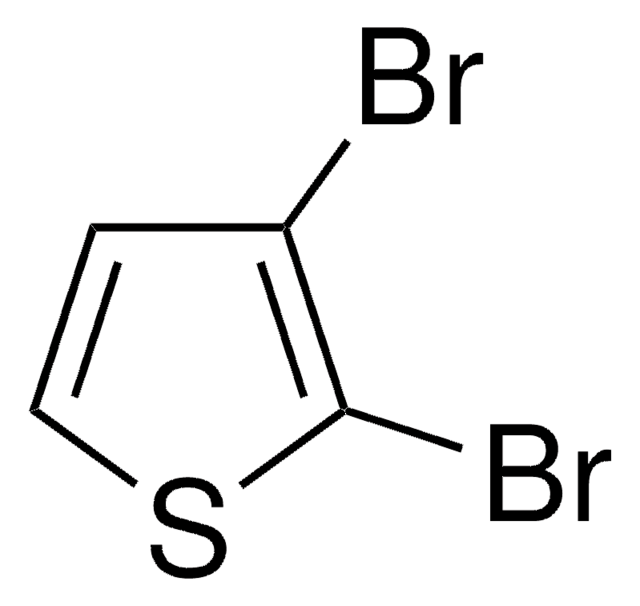
3,4-Dibromothiophene
99%
Synonym(s):
3,4-Dibromothiophene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H2Br2S
CAS Number:
Molecular Weight:
241.93
Beilstein:
107642
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.640 (lit.)
bp
221-222 °C (lit.)
mp
4-5 °C (lit.)
density
2.188 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Brc1cscc1Br
InChI
1S/C4H2Br2S/c5-3-1-7-2-4(3)6/h1-2H
InChI key
VGKLVWTVCUDISO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Double photoionization spectra of 3,4-dibromothiophene has been investigated by coincidence spectroscopy technique based on electron time-of-flight measurement.
Application
3,4-Dibromothiophene was used in the preparation of thieno[3,4-b]thiophene. It was aslo used as starting material in the synthesis of alkyl substituted, fused thiophenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Linusson et al.
The Journal of chemical physics, 129(23), 234303-234303 (2008-12-24)
We report the double photoionization spectra of thiophene, 3-bromothiophene, and 3,4-dibromothiophene using a coincidence spectroscopy technique based on electron time-of-flight measurements. Spectra have been recorded between the onset and 40.814 eV using He IIalpha radiation. The He I photoelectron spectrum
An Alternative Synthysis of Thieno [3, 4-b] Thiophene.
Brandsma L and Verkruijsse HD.
Synthetic Communications, 20(15), 2275-2277 (1990)
Mingqian He et al.
The Journal of organic chemistry, 72(2), 442-451 (2007-01-16)
We have established a series of synthetic methods to synthesize alkyl-substituted fused thiophenes with degrees of fusion from two to seven rings. These fused thiophene ring compounds have very good solubility in common organic solvents, making possible the solution processing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service