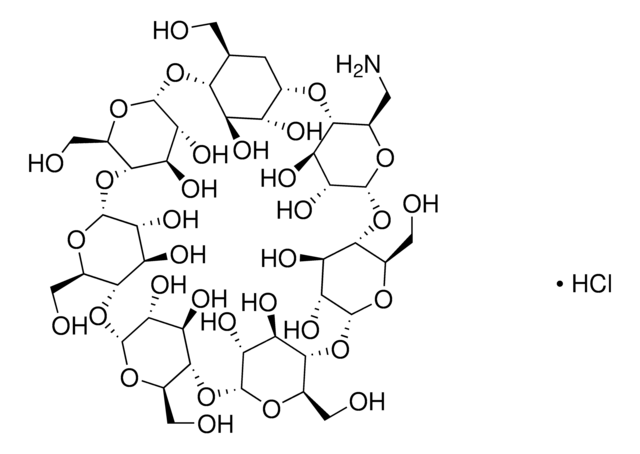
The molecular weight for the β-Cyclodextrin moiety is 1135 g/mole and the contribution from the succinic acid portion is 100.1 g/mole. This mass is used for succinic acid as water is lost during the condensation reaction when cyclodextrin and succinic acid form the conjugate. Thus, the average molecular formula and average formula weight can be expressed as the following. C42H70-nO35 • (C4H5O3)n or 1135.0 + n •100.1 The average degree of substitution for this product is ~3.5 for this product making the molecular weight approximately ~1485 g/mole for this product. The degree of substitution can vary from 2.5 - 5 for this product and the batch specific certificate of analysis should be reviewed to gather the degree of substitution (n).
크기 선택
About This Item
추천 제품
양식
powder
불순물
~5% water
색상
white
mp
225 °C ((437 °F ) - Decomposes on heating)
저장 온도
−20°C
SMILES string
CC(=O)CCC(=O)OC[C@H]1O[C@@H]2O[C@H]3[C@@H](O)[C@H](O)[C@H](O[C@@H]3COC(=O)CCC(O)=O)O[C@H]4[C@@H](O)[C@H](O)[C@H](O[C@@H]4COC(=O)CCC(O)=O)O[C@H]5[C@@H](O)[C@H](O)[C@H](O[C@@H]5COC(=O)CCC(O)=O)O[C@H]6[C@@H](O)[C@H](O)[C@H](O[C@@H]6COC(=O)CCC(O)=O)O[C@H]7[C@@H](O)[C@H](O)[C@H](O[C@@H]7COC(=O)CCC(O)=O)O[C@H]8[C@@H](O)[C@H](O)[C@H](O[C@@H]8COC(=O)CCC(O)=O)O[C@H]1[C@@H](O)[C@@H]2O
InChI
1S/C71H100O55/c1-23(72)2-9-37(85)106-16-24-58-44(92)51(99)65(113-24)121-59-25(17-107-38(86)10-3-31(73)74)115-67(53(101)46(59)94)123-61-27(19-109-40(88)12-5-33(77)78)117-69(55(103)48(61)96)125-63-29(21-111-42(90)14-7-35(81)82)119-71(57(105)50(63)98)126-64-30(22-112-43(91)15-8-36(83)84)118-70(56(104)49(64)97)124-62-28(20-110-41(89)13-6-34(79)80)116-68(54(102)47(62)95)122-60-26(18-108-39(87)11-4-32(75)76)114-66(120-58)52(100)45(60)93/h24-30,44-71,92-105H,2-22H2,1H3,(H,73,74)(H,75,76)(H,77,78)(H,79,80)(H,81,82)(H,83,84)/t24-,25-,26-,27-,28-,29-,30-,44+,45+,46+,47+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-/m1/s1
InChI key
DIRLEDPEXJLCIL-JCWBWLHSSA-N
일반 설명
애플리케이션
Succinyl-β-cyclodextrin and carboxymethyl-β-cyclodextrin are used as chiral selective agents in capillary electrophoresis for the separation of di- and tri-peptide enantiomers and catechin enantiomers. Succinyl-β-cyclodextrin is used to optimize analysis of PNA-DNA duplexes with diethylthiadicarbocyanine dye.
기타 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.