This product can be dissolved in water and acetonitrile. However, specific saturation concentration information is not available. The product is indicated as not usable for in vitro cell experiments. Please check Section 3 Use of Products on the webpage of Terms and Conditions: https://www.sigmaaldrich.com/life-science/legal/terms-and-conditions#3
PHR1701
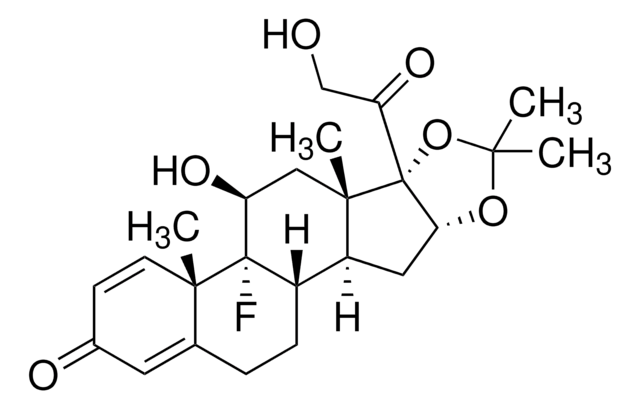
Triamcinolone Acetonide
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
Triamcinolone acetonide, 9α-Fluoro-11β,16α,17α,21-tetrahydroxy-1,4-pregnadiene-3,20-dione 16,17-acetonide, 9α-Fluoro-16α-hydroxyprednisolone 16α,17α-acetonide
크기 선택
About This Item
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. T1900000
traceable to USP 1677002
API family
triamcinolone
CofA
current certificate can be downloaded
포장
pkg of 1 g
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
[H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1(F)[C@@H](O)C[C@@]4(C)[C@@]2([H])C[C@H]5OC(C)(C)O[C@@]45C(=O)CO
InChI
1S/C24H31FO6/c1-20(2)30-19-10-16-15-6-5-13-9-14(27)7-8-21(13,3)23(15,25)17(28)11-22(16,4)24(19,31-20)18(29)12-26/h7-9,15-17,19,26,28H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,21-,22-,23-,24+/m0/s1
InChI key
YNDXUCZADRHECN-JNQJZLCISA-N
유전자 정보
human ... NR3C1(2908)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Triamcinolone Acetonide is a glucocorticoid[1] and a derivative of pregnane, used mainly in the treatment of rheumatic disease, allergic and dermatologic symptoms.[2]
애플리케이션
분석 메모
기타 정보
각주
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
-
What is the solubility of this? In which solvents and up to what concentration can it dissolve? Is it usable in vitro cell experiments?
1 답변-
도움이 되었습니까?
-
-
How do you know that this product is working and about how long.
1 답변-
The product is assigned an expiration date of approximately 4 years from the date of release. The stability of this compound in solution has not been determined. Note that this item is intended for research use only and is not suitable for human or veterinary use.
도움이 되었습니까?
-
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.