W237809
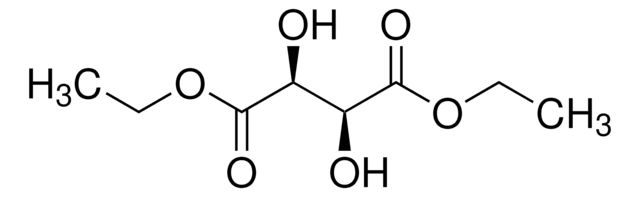
Diethyl L-tartrate
≥99%, FG
동의어(들):
(+)-Diethyl L-tartrate, L-(+)-Tartaric acid diethyl ester
About This Item
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Fragrance grade
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
규정 준수
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
분석
≥99%
광학 활성
[α]20/D +8.5°, neat
refractive index
n20/D 1.446 (lit.)
bp
280 °C (lit.)
density
1.204 g/mL at 25 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
향수 알레르기항원
no known allergens
감각 수용성의
fruity; wine-like
SMILES string
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
InChI key
YSAVZVORKRDODB-PHDIDXHHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
199.4 °F - closed cup
Flash Point (°C)
93 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.