추천 제품
Quality Level
분석
97%
refractive index
n20/D 1.396 (lit.)
bp
142 °C (lit.)
density
0.885 g/mL at 25 °C (lit.)
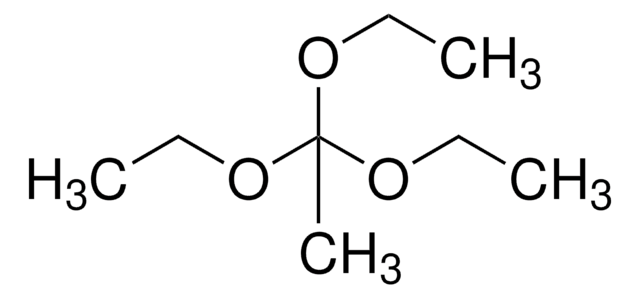
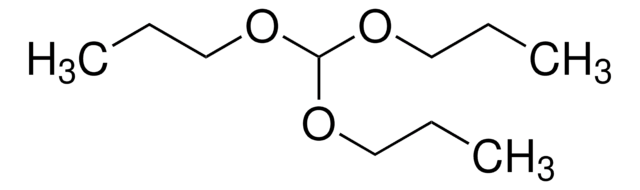
SMILES string
CCOC(C)(OCC)OCC
InChI
1S/C8H18O3/c1-5-9-8(4,10-6-2)11-7-3/h5-7H2,1-4H3
InChI key
NDQXKKFRNOPRDW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Triethyl orthoacetate can be used for the synthesis of:
- Ordered mesoporous carbons for catalysis, electrochemistry and hydrogen storage applications.
- Esters of phosphonic acids, carboxylic acids and phosphinic acids.
- Ethyl alk-4-enoates by Johnson–Claisen rearrangement reaction.
- Benzoxazoles, benzimidazoles and oxazolo[4,5-b]pyridines.
- Quinazolin-4(3H)-one derivatives.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
102.2 °F - Non-equilibrium method
Flash Point (°C)
39 °C - Non-equilibrium method
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis of ordered mesoporous carbons with channel structure from an organic-organic nanocomposite
Tanaka S, et al.
Chemical Communications (Cambridge, England), 16, 2125-2127 (2005)
Treatment of Baylis-Hillman adducts with triethyl orthoacetate in the presence of heterogeneous catalysts: a method for the stereoselective synthesis of two different types of trisubstituted alkenes
Das B, et al.
Tetrahedron Letters, 47(43), 7619-7623 (2006)
Silica sulfuric acid catalyzed synthesis of benzoxazoles, benzimidazoles and oxazolo [4, 5-b] pyridines under heterogeneous and solvent-free conditions
Mohammadpoor-Baltork I, et al.
Journal of the Iranian Chemical Society, 5(1), S65-S70 (2008)
A new approach to the facile synthesis of mono-and disubstituted quinazolin-4 (3H)-ones under solvent-free conditions.
Salehi P, et al.
Tetrahedron Letters, 46(41), 7051-7053 (2005)
Efficient esterification of carboxylic acids and phosphonic acids with trialkyl orthoacetate in ionic liquid
Yoshino T, et al.
Tetrahedron, 62(6), 1309-1317 (2006)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.