추천 제품
vapor density
5.52 (vs air)
vapor pressure
1 mmHg ( 40 °C)
제품 라인
ReagentPlus®
분석
99%
형태
liquid
refractive index
n20/D 1.413 (lit.)
bp
199 °C (lit.)
mp
−51-−50 °C (lit.)
density
1.055 g/mL at 25 °C (lit.)
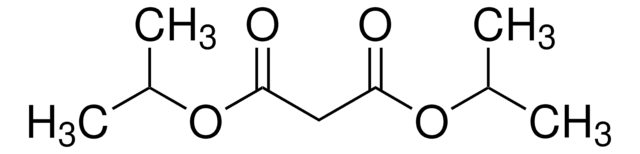
SMILES string
CCOC(=O)CC(=O)OCC
InChI
1S/C7H12O4/c1-3-10-6(8)5-7(9)11-4-2/h3-5H2,1-2H3
InChI key
IYXGSMUGOJNHAZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Diethyl malonate is diethyl ester of malonic acid. It is widely used as a versatile building block for introducing the malonate functional group into molecules. Acylation of diethyl malonate using magnesium chloride and triethylamine is reported. K2CO3-catalyzed 1,4-addition reaction of diethyl malonate with various substituted 1,2-allenic ketones yields polyfunctionalized β,γ-unsaturated enones.
애플리케이션
Diethyl malonate was used to investigate Knoevenagel condensation reactions in microreactor using zeolite catalysts obtained by grafting amino groups onto NaX and CsNaX zeolites. It may be used in the synthesis of diethyl benzylidenemalonate by base catalyzed Knoevenagel condensation with benzaldehyde. It may be used in the synthesis of α-aryl malonates.
포장
Packaged in glass bottles
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
199.4 °F - closed cup
Flash Point (°C)
93 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Shengming Ma et al.
The Journal of organic chemistry, 68(23), 8996-9002 (2003-11-08)
The K2CO3 (10 mol %)-catalyzed 1,4-addition reaction of diethyl malonate with various substituted 1,2-allenic ketones leading to polyfunctionalized beta,gamma-unsaturated enones 3 or 4 was studied. With 3-unsubstituted 1-substituted-1,2-allenyl ketones, the highly selective formation of beta,gamma-unsaturated enones 4 was observed; with
Solvent-free organocatalytic Michael addition of diethyl malonate to nitroalkenes: the practical synthesis of Pregabalin and ?-nitrobutyric acid derivatives
J Liu, et al.
Tetrahedron, 67, 636-640 (2011)
Edward J Hennessy et al.
Organic letters, 4(2), 269-272 (2002-02-14)
[reaction: see text] A general method for the synthesis of alpha-aryl malonates is described. The coupling of an aryl iodide and diethyl malonate in the presence of Cs(2)CO(3) and catalytic amounts of copper(I) iodide and 2-phenylphenol affords the alpha-aryl malonate
Integration of heterogeneous catalysts into complex synthetic routes: sequential vs. one-pot reactions in a (Knoevenagel+ Mukaiyama-Michael+ hydrogenation+ transesterification) sequence.
Fraile JM, et al.
Catalysis Science & Technology, 3(2), 436-443 (2013)
Lian Jin Liu et al.
Nucleosides, nucleotides & nucleic acids, 30(10), 784-797 (2011-10-05)
Novel 5'-norcarbocyclic adenosine phosphonic acid analogues with 6'-electropositive moiety such as spirocyclopropane were designed and synthesized from the commercially available diethylmalonate 5. Regioselective Mitsunobu reaction proceeded in the presence of an allylic functional group at a low reaction temperature in
문서
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.