추천 제품
분석
98%
형태
powder
bp
110-115 °C/15 mmHg (lit.)
mp
62-70 °C (lit.)
SMILES string
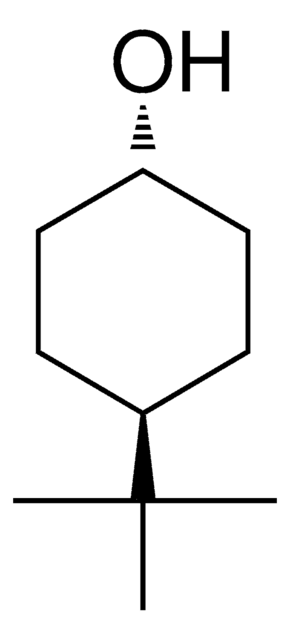
CC(C)(C)C1CCC(O)CC1
InChI
1S/C10H20O/c1-10(2,3)8-4-6-9(11)7-5-8/h8-9,11H,4-7H2,1-3H3
InChI key
CCOQPGVQAWPUPE-UHFFFAOYSA-N
애플리케이션
4-tert-Butylcyclohexanol (mixture of cis and trans) can be used as a reactant to synthesize tris(4,4′-di-tert-butyl-2,2′-bipyridine)(trans-4-tert-butylcyclohexanolato)deca-μ-oxido-heptaoxidoheptavanadium oxide cluster complex by reacting with [V8O20(C18H24N2)4]. It can also be used as a reactant in competitive Oppenauer oxidation experiments in the presence of zeolite BEA as a stereoselective catalyst. Only cis-isomer is selectively converted to the corresponding ketone, whereas trans-isomer remains unchanged.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
221.0 °F - closed cup
Flash Point (°C)
105 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Tris (4, 4?-di-tert-butyl-2, 2?-bipyridine)(trans-4-tert-butylcyclohexanolato) deca- ? -oxido-heptaoxidoheptavanadium acetonitrile monosolvate including another unknown solvent molecule
Kodama S, et al.
IUCrData, 5(4), x200449-x200449 (2020)
Stereoselective Meerwein-Ponndorf-Verley and Oppenauer reactions catalysed by zeolite BEA
Creyghton EJ, et al.
J. Mol. Catal. A: Chem., 115(3), 457-472 (1997)
Mireia Oromí-Farrús et al.
Journal of analytical methods in chemistry, 2012, 452949-452949 (2012-06-01)
The use of iodine as a catalyst and either acetic or trifluoroacetic acid as a derivatizing reagent for determining the enantiomeric composition of acyclic and cyclic aliphatic chiral alcohols was investigated. Optimal conditions were selected according to the molar ratio
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.