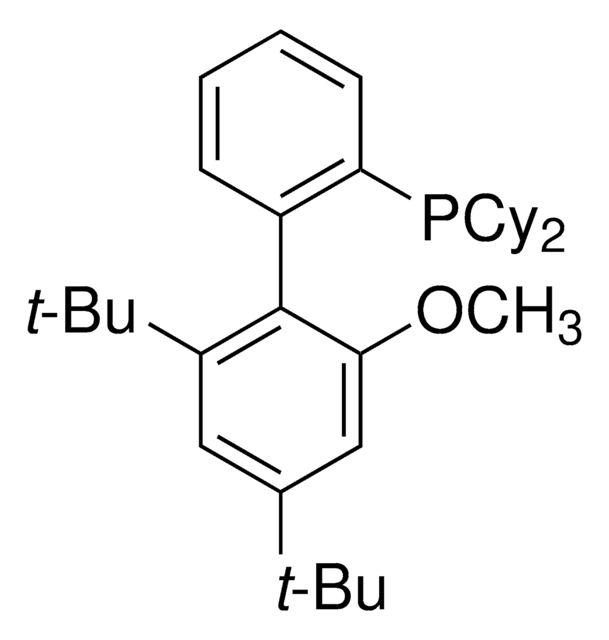
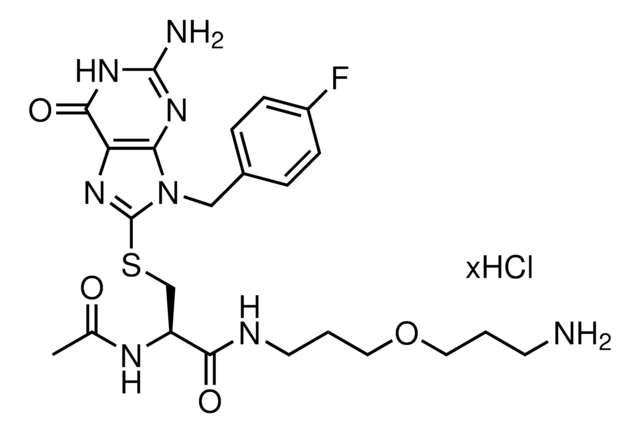
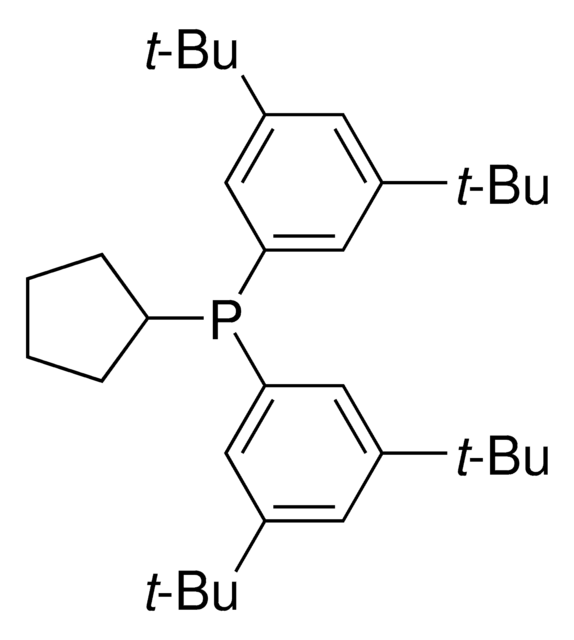
929328
FBnG-C3-PEG5-C3-NH2 hydrochloride
≥95%
동의어(들):
(R)-2-acetamido-3-((2-amino-9-(4-fluorobenzyl)-6-oxo-6,9-dihydro-1H-purin-8-yl)thio)-N-(19-amino-4,7,10,13,16-pentaoxanonadecyl)propanamide hydrochloride
크기 선택
About This Item
추천 제품
분석
≥95%
양식
powder
작용기
amine
저장 온도
2-8°C
SMILES string
O=C1NC(N)=NC2=C1N=C(SC[C@@H](C(NCCCOCCOCCOCCOCCOCCCN)=O)NC(C)=O)N2CC3=CC=C(C=C3)F.Cl
애플리케이션
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
기타 정보
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.