909602
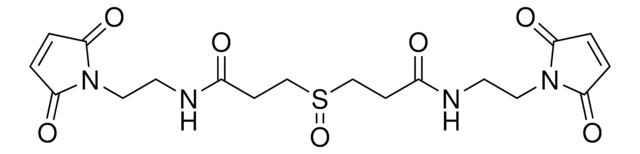
DSSO crosslinker
≥95%
동의어(들):
Bis(2,5-dioxopyrrolidin-1-yl) 3,3′-sulfinyldipropionate, Bis-(propionic acid NHS ester)-sulfoxide, Mass spectrometry-cleavable crosslinker for studying protein-protein interations
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C14H16N2O9S
CAS Number:
Molecular Weight:
388.35
MDL number:
UNSPSC 코드:
12161502
추천 제품
분석
≥95%
형태
powder
재고 정보
available only in USA
저장 온도
2-8°C
애플리케이션
DSSO (disuccinimidyl sulfoxide) crosslinker is a homobifunctional, amine-targeting, sulfoxide-containing crosslinker for analysis of protein-protein interactions (PPIs) through crosslinking mass spectrometry (XL-MS). Membrane-permeable DSSO possesses two N-hydroxysuccinimide (NHS) esters for targeting Lys, a 10.1 Å spacer arm, and two symmetrical C-S cleavable bonds adjacent to the central sulfoxide. The post-cleavage spacer yields tagged peptides for unambiguous identification by collision-induced dissociation in tandem MS. DSSO Crosslinker provides complementary data to thiol-reactive and acidic residue-targeting reagents and will find wide utility in the elucidation of PPIs, study of proteins that function as complexes, quantification of structural dynamics, and the quest for targeting ″undruggable″ protein targets.
기타 정보
Technology Spotlight: Cross-Linkers for Elucidation of Protein-Protein Interactions
Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes
Structural dynamics of the human COP9 signalosome revealed by cross-linking mass spectrometry and integrative modeling
Developing a Multiplexed Quantitative Cross-Linking Mass Spectrometry Platform for Comparative Structural Analysis of Protein Complexes
Development of a Novel Sulfoxide-Containing MS-Cleavable Homobifunctional Cysteine-Reactive Cross-Linker for Studying Protein–Protein Interactions
Developing an Acidic Residue Reactive and Sulfoxide-Containing MS-Cleavable Homobifunctional Cross-Linker for Probing Protein-Protein Interactions
Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes
Structural dynamics of the human COP9 signalosome revealed by cross-linking mass spectrometry and integrative modeling
Developing a Multiplexed Quantitative Cross-Linking Mass Spectrometry Platform for Comparative Structural Analysis of Protein Complexes
Development of a Novel Sulfoxide-Containing MS-Cleavable Homobifunctional Cysteine-Reactive Cross-Linker for Studying Protein–Protein Interactions
Developing an Acidic Residue Reactive and Sulfoxide-Containing MS-Cleavable Homobifunctional Cross-Linker for Probing Protein-Protein Interactions
법적 정보
Subject to US Patent #9,222,943 and US Patent Application #15/275,001 of the Regents of the University of California
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Tara K Bartolec et al.
Analytical chemistry, 92(2), 1874-1882 (2019-12-19)
Saccharomyces cerevisiae has the most comprehensively characterized protein-protein interaction network, or interactome, of any eukaryote. This has predominantly been generated through multiple, systematic studies of protein-protein interactions by two-hybrid techniques and of affinity-purified protein complexes. A pressing question is to
Clinton Yu et al.
Analytical chemistry, 88(20), 10301-10308 (2016-10-19)
Cross-linking mass spectrometry (XL-MS) represents a recently popularized hybrid methodology for defining protein-protein interactions (PPIs) and analyzing structures of large protein assemblies. In particular, XL-MS strategies have been demonstrated to be effective in elucidating molecular details of PPIs at the
Lei Lu et al.
Journal of proteome research, 17(7), 2370-2376 (2018-05-26)
Protein chemical cross-linking combined with mass spectrometry has become an important technique for the analysis of protein structure and protein-protein interactions. A variety of cross-linkers are well developed, but reliable, rapid, and user-friendly tools for large-scale analysis of cross-linked proteins
Daniela-Lee Smith et al.
Analytical chemistry, 90(15), 9101-9108 (2018-07-14)
This study investigated the enzyme-substrate interaction between Saccharomyces cerevisiae arginine methyltransferase Hmt1p and nucleolar protein Npl3p, using chemical cross linking/mass spectrometry (XL/MS). We show that XL/MS can capture transient interprotein interactions that occur during the process of methylation, involving a
Athit Kao et al.
Molecular & cellular proteomics : MCP, 10(1), M110-M110 (2010-08-26)
Knowledge of elaborate structures of protein complexes is fundamental for understanding their functions and regulations. Although cross-linking coupled with mass spectrometry (MS) has been presented as a feasible strategy for structural elucidation of large multisubunit protein complexes, this method has
문서
Sulfoxide-containing MS-cleavable cross-linkers capture protein-protein interactions in native cellular environments, aiding PPI identification.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.