755745
2,2′-Azobis(2-methylpropionitrile)
recrystallized from methanol, 99%
동의어(들):
α,α′-Azoisobutyronitrile, AIBN, Azobisisobutyronitrile, Free radical initiator
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
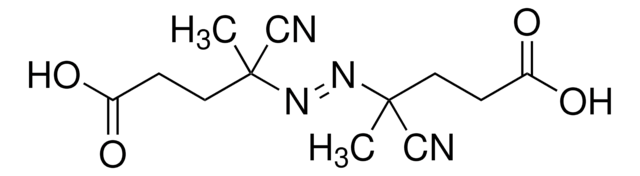
(CH3)2C(CN)N=NC(CH3)2CN
CAS Number:
Molecular Weight:
164.21
Beilstein:
1708400
EC Number:
MDL number:
UNSPSC 코드:
12162002
PubChem Substance ID:
NACRES:
NA.23
추천 제품
Quality Level
분석
99%
형태
crystals
mp
102-104 °C (dec.) (lit.)
103-107 °C
저장 온도
−20°C
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
This AIBN was recrystallized and is ready for use as a polymerization initiator.
애플리케이션
- Porous Acid-Base Hybrid Polymers for Enhanced NH3 Uptake: This study discusses the use of 2,2′-Azobis(2-methylpropionitrile) in the synthesis of acid-base hybrid polymers, highlighting its role in enhancing ammonia uptake through cooperative hydrogen bonds (X Luo, Y Liu, et al., 2023).
- Extraction of Fluoroquinolones from Milk: The development of molecularly imprinted polymers using 2,2′-Azobis(2-methylpropionitrile) as an initiator for the extraction of antibiotics from milk showcases its application in food safety and pharmaceutical analysis (E Megias-Pérez, et al., 2023).
- Thermo-responsive Copolymer Visible Light Catalyst: Highlighting the use of 2,2′-Azobis(2-methylpropionitrile) in the synthesis of thermo-responsive copolymers, this study explores its applications in catalysis and material science, particularly in photoreactive polymers (S Wu, et al., 2024).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Self-react. C
보충제 위험성
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point (°F)
122.0 °F
Flash Point (°C)
50 °C
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Stana Kovačević et al.
Polymers, 11(5) (2019-05-30)
The objective of this research was to verify the feasibility of the use of newly synthesized biopolymer materials for sizing cotton yarns based on the basic principles of chemical modification. Research included acid hydrolysis of potato starch up to controlled
Wenwen Li et al.
Macromolecular rapid communications, 32(1), 74-81 (2011-03-25)
Amphiphilic star shaped polymers with poly(ethylene oxide) (PEO) arms and cross-linked hydrophobic core were synthesized in water via either conventional free radical polymerization (FRP) or atom transfer radical polymerization (ATRP) techniques using a simple "arm-first" method. In FRP, PEO based
Lianghui Liu et al.
Organic letters, 14(22), 5692-5695 (2012-10-31)
In the presence of a catalytic amount of radical initiator AIBN, primary amines are oxidatively coupled to imines and tertiary amines are cyanated to α-aminonitriles. These "metal-free" aerobic oxidative coupling reactions may find applications in a wide range of "green"
Mathieu Le Noir et al.
Journal of separation science, 32(9), 1471-1479 (2009-04-29)
Highly efficient removal of endocrine-disrupting compounds (EDCs) such as 17beta-estradiol (E2), 4-nonylphenol (NP) and atrazine from water was achieved using a novel macroporous adsorption medium. The medium consisted of a macroporous poly(vinyl alcohol) (PVA) cryogel with molecularly imprinted polymer (MIP)
Idil Ipek Yilmaz et al.
Macromolecular rapid communications, 33(9), 856-862 (2012-04-21)
Polymers containing maleimide groups on their side chains have been synthesized by utilization of a novel styrenic monomer containing a masked-maleimide unit. AIBN initiated free radical polymerization and reverse addition-fragmentation chain transfer (RAFT) polymerization was utilized for synthesis of copolymers
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.