About This Item
추천 제품
분석
97%
양식
sublimed
손실
0.5 wt. %, 280 °C
반도체 특성
P-type (mobility=0.3 cm2/V·s)
SMILES string
c1cc2cc3cc4cc5sccc5cc4cc3cc2s1
InChI
1S/C18H10S2/c1-3-19-17-9-15-8-14-6-12-2-4-20-18(12)10-16(14)7-13(15)5-11(1)17/h1-10H
InChI key
DAMUWSYTQPWFIY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
가장 최신 버전 중 하나를 선택하세요:
문서
Organic materials in optoelectronic devices like LEDs and solar cells are of significant academic and commercial interest.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.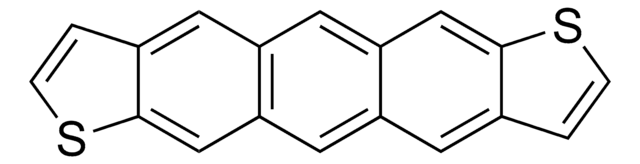
![Dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene sublimed grade, 99%](/deepweb/assets/sigmaaldrich/product/structures/196/451/8a650b8e-abbb-4ef1-9be4-73f223165062/640/8a650b8e-abbb-4ef1-9be4-73f223165062.png)



![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)



