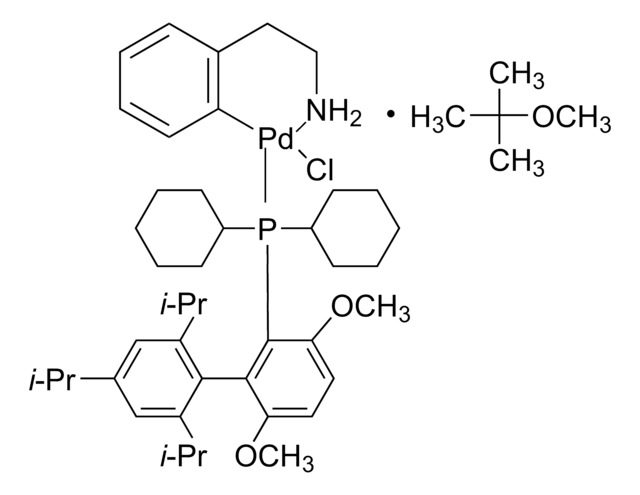
707589
RuPhos Pd G1 Methyl t-Butyl Ether Adduct
95%
동의어(들):
(RuPhos) palladium(II) phenethylamine chloride (1:1 MTBE solvate), Chloro-(2-Dicyclohexylphosphino-2′,6′-diisopropoxy-1,1′-biphenyl)[2-(2-aminoethyl)phenyl]palladium(II) - methyl-t-butyl ether adduct, RuPhos Palladacycle, RuPhos precatalyst
About This Item
추천 제품
Quality Level
분석
95%
양식
solid
특징
generation 1
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
180-200 °C
작용기
phosphine
SMILES string
COC(C)(C)C.NCCc1ccccc1[Pd]Cl.CC(C)Oc2cccc(OC(C)C)c2-c3ccccc3P(C4CCCCC4)C5CCCCC5
InChI
1S/C30H43O2P.C8H10N.C5H12O.ClH.Pd/c1-22(2)31-27-19-13-20-28(32-23(3)4)30(27)26-18-11-12-21-29(26)33(24-14-7-5-8-15-24)25-16-9-6-10-17-25;9-7-6-8-4-2-1-3-5-8;1-5(2,3)6-4;;/h11-13,18-25H,5-10,14-17H2,1-4H3;1-4H,6-7,9H2;1-4H3;1H;/q;;;;+1/p-1
InChI key
OMMPYFRVDWZBNR-UHFFFAOYSA-M
애플리케이션
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.