663107
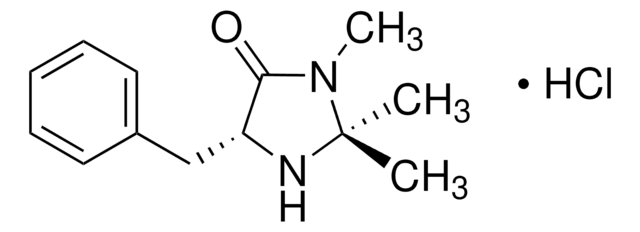
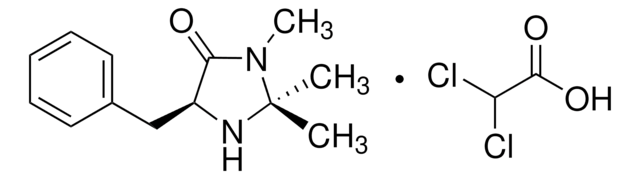
(2S,5S)-(−)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone
97%
동의어(들):
(2S,5S)-2-tert-Butyl-3-methyl-5-phenylmethyl-4-imidazolidinone, (2S,5S)-5-Benzyl-2-tert-butyl-3-methyl-4-imidazolidinone
크기 선택
About This Item
추천 제품
분석
97%
양식
solid
mp
93-100 °C (lit.)
작용기
phenyl
SMILES string
CN1[C@H](N[C@@H](Cc2ccccc2)C1=O)C(C)(C)C
InChI
1S/C15H22N2O/c1-15(2,3)14-16-12(13(18)17(14)4)10-11-8-6-5-7-9-11/h5-9,12,14,16H,10H2,1-4H3/t12-,14-/m0/s1
InChI key
SKHPYKHVYFTIOI-JSGCOSHPSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- The chiral transformation reaction, including Friedel-Crafts and Mukaiyama-Michael reactions.[2]
- The preparation of substituted spiroundecenetriones via asymmetric domino Knoevenagel/Diels-Alder reactions.[3]
- The asymmetric synthesis of β-hydroxy aldehydes and their dimethylacetals via aldehyde-aldehyde aldol condensation reaction.[4]
- The enantioselective α-fluorination of aldehydes using N-fluorobenzenesulfonamide as a fluorinating agent.[5]
- The stereoselective preparation of (oxomethyl)oxabicyclo[3.2.1]octenones and tricyclic pyrroles via [4+3] cycloaddition of (trialkylsiloxy)pentadienals to furans.[6]
특징 및 장점
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Extraordinary functional group tolerance
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
문서
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)