모든 사진(1)
크기 선택
보기 변경
1 G
₩38,063
5 G
₩94,903
About This Item
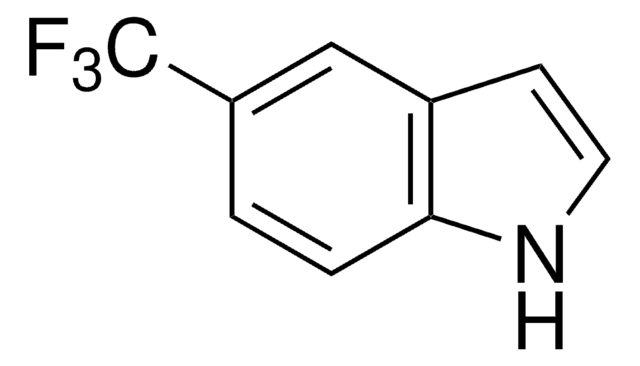
실험식(Hill 표기법):
C10H9NO2
CAS Number:
Molecular Weight:
175.18
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
mp
126-128 °C (lit.)
작용기
ester
SMILES string
COC(=O)c1ccc2[nH]ccc2c1
InChI
1S/C10H9NO2/c1-13-10(12)8-2-3-9-7(6-8)4-5-11-9/h2-6,11H,1H3
InChI key
DRYBMFJLYYEOBZ-UHFFFAOYSA-N
일반 설명
애플리케이션
Methyl indole-5-carboxylate may be used as a reactant in the following processes:
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Fei Yang et al.
Organic letters, 12(22), 5214-5217 (2010-10-23)
A novel cross dehydrogenative coupling (CDC) reaction of N,N-dimethylanilines with methyl ketones by cooperative copper and aminocatalysis has been developed, which leads to the formation of β-arylamino ketones in 42-73% yields. Moreover, the copper-catalyzed alkylation of free (NH) indoles with
Wang, T. C.; et al.
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 21, 1407-1407 (2010)
Liu, Z.; et al.
Letters in Organic Chemistry, 7, 666-666 (2010)
Wen-Jie Lu et al.
European journal of medicinal chemistry, 64, 498-511 (2013-05-21)
This report describes the synthesis, and in vitro and in vivo antimalarial evaluations of certain ester-modified neocryptolepine (5-methyl-5H-indolo[2,3-b]quinoline) derivatives. The modifications were carried out by introducing ester groups at the C2 and/or C9 position on the neocryptolepine core and the
A facial synthesis and antimicrobial activity of some pyrazole derivatives carrying indole.
Sarma KN, et al.
Journal of Chemistry, 7(3), 745-750 (2010)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.