456764
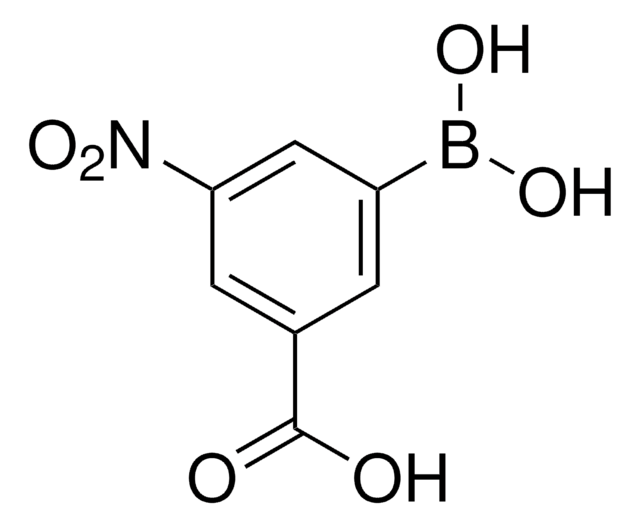
3-Carboxyphenylboronic acid
≥95%
동의어(들):
μ-Carboxyphenylboronic acid, 3-(Dihydroxyborane)benzoic acid, 3-(Dihydroxyboryl)benzoic acid, 3-Boronobenzoic acid, 3-Carboxybenzeneboronic acid
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
HO2CC6H4B(OH)2
CAS Number:
Molecular Weight:
165.94
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95%
mp
243-247 °C (lit.)
작용기
carboxylic acid
SMILES string
OB(O)c1cccc(c1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10)
InChI key
DBVFWZMQJQMJCB-UHFFFAOYSA-N
애플리케이션
3-Carboxyphenylboronic acid can be used as a substrate in the preparation of:
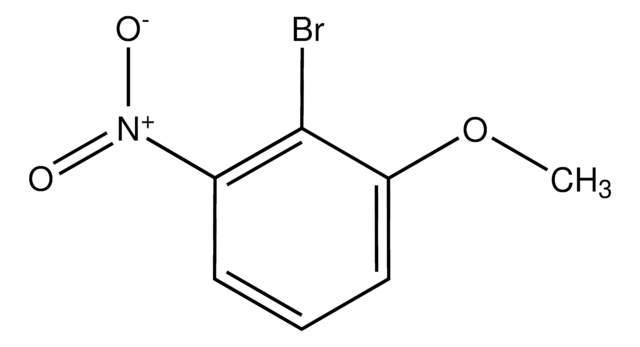
- Biaryl derivatives by reacting with bromoaniline through the Suzuki-Miyaura coupling reaction.
- Boronic acid-functionalized block copolymer.
- 1H-Imidazo[1,2-a]quinoxaline derivatives.
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Novel rhodamine dyes via Suzuki coupling of xanthone triflates with arylboroxins
Calitree, B. D.; Detty, M. R.
Synlett, 89-92 (2010)
Jumin Yang et al.
Materials science & engineering. C, Materials for biological applications, 116, 111250-111250 (2020-08-19)
Various nanoparticles as drug delivery system provide significant improvements in the cancer treatment. However, their clinical success remains elusive in large part due to their inability to overcome both systemic and tumor tissue barriers. The nanosystems with nanoproperty-transformability (surface, size
Christopher G Barber et al.
Bioorganic & medicinal chemistry letters, 14(12), 3227-3230 (2004-05-20)
A series of 1-isoquinolinylguanidines are shown to be potent inhibitors of uPA with selectivity over tPA and plasmin. Potency is enhanced by the presence of a 4-halo and a 7-aryl substituent, particularly when substituted by a 3-carboxylic acid group. Compound
Heterocycles, 60, 1891-1897 (2003)
New imidazo [1, 2-a] quinoxaline derivatives: synthesis and in vitro activity against human melanoma
Deleuze-Masquefa C, et al.
European Journal of Medicinal Chemistry, 44(9), 3406-3411 (2009)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.