모든 사진(1)
About This Item
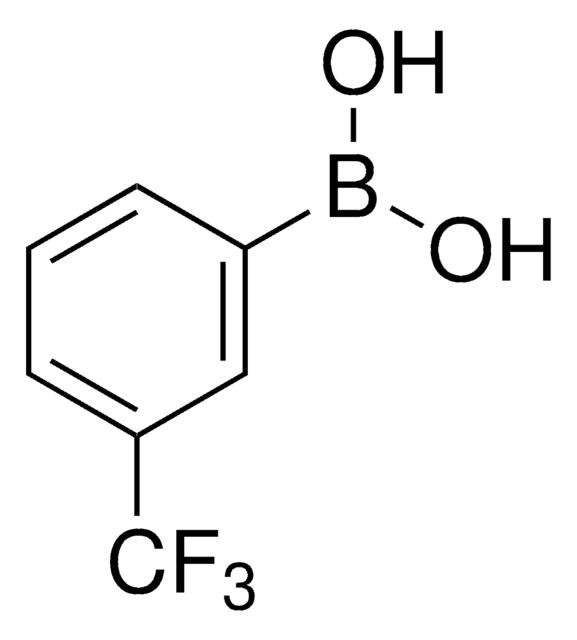
Linear Formula:
CF3C6H4B(OH)2
CAS Number:
Molecular Weight:
189.93
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
445193 제품과 관련한 질문은 현지 머크 영업소 또는 판매 사원에게 문의해 주십시오. 고객지원팀으로 연락바랍니다.
추천 제품
분석
≥95.0%
양식
solid
mp
111-114 °C (lit.)
작용기
fluoro
SMILES string
OB(O)c1ccccc1C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-3-1-2-4-6(5)8(12)13/h1-4,12-13H
InChI key
JNSBEPKGFVENFS-UHFFFAOYSA-N
애플리케이션
2-(Trifluoromethyl)phenylboronic acid can be used as a reactant:
- In Suzuki-coupling reactions to prepare 2-trifluoromethyl aryl or heteroaryl derivatives.[1]
- To synthesize 4-(2-trifluoromethyl)phenylpyrrolo[2,3-d]pyrimidine as a potential antagonist of corticotropin-releasing hormone.[2]
- To prepare 2-nitro-6-(trifluoromethyl)phenylboronic acid by nitration reaction.[3]
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Studies of palladium-catalyzed coupling reactions for preparation of hindered 3-arylpyrroles relevant to (-)-rhazinilam and its analogues
Ghosez L, et al.
Canadian Journal of Chemistry, 79(11), 1827-1839 (2001)
Efficient synthetic approach to heterocycles possessing the 3, 3-disubstituted-2, 3-dihydrobenzofuran skeleton via diverse palladium-catalyzed tandem reactions
Szlosek-Pinaud M, et al.
Tetrahedron, 63(16), 3340-3349 (2007)
Functionalization of pyrrolo [2, 3-d] pyrimidine by palladium-catalyzed cross-coupling reactions
Tumkevicius, S and Dodonova, J
Chemistry of Heterocyclic Compounds, 48(2), 258-279 (2012)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.