모든 사진(1)
About This Item
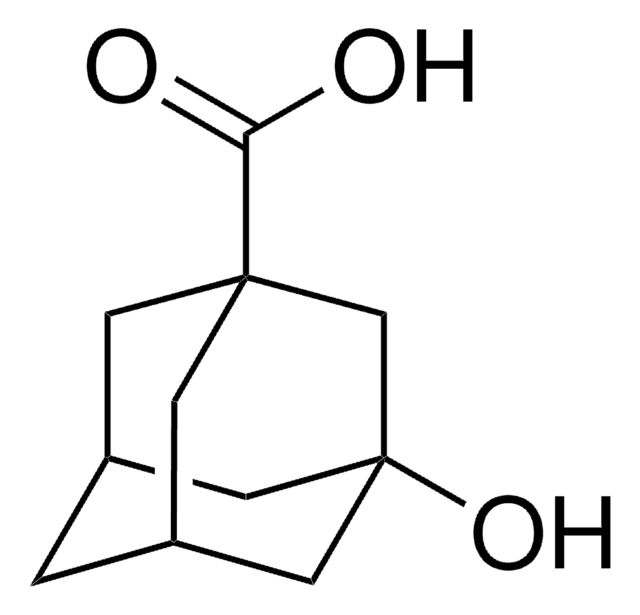
실험식(Hill 표기법):
C10H14O2
CAS Number:
Molecular Weight:
166.22
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
양식
solid
mp
>300 °C (lit.)
작용기
hydroxyl
ketone
SMILES string
O[C@]12C[C@@H]3C[C@H](C1)C(=O)[C@@H](C3)C2
InChI
1S/C10H14O2/c11-9-7-1-6-2-8(9)5-10(12,3-6)4-7/h6-8,12H,1-5H2/t6-,7-,8+,10-
InChI key
TZBDEVBNMSLVKT-XYYXLIQBSA-N
일반 설명
애플리케이션
5-Hydroxy-2-adamantanone may be used in the following studies:
- As a model compound to investigate the application of lanthanide NMR shift reagents for the analysis of disubstituted derivative of adamantane.[1]
- As a starting material for the synthesis of E-2-amino-5-hydroxyadamantane.[3]
- As a starting material for the synthesis of 4-(triphenylsilyloxy)adamantan-1-ol.[4]
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
S S Boĭko et al.
Farmakologiia i toksikologiia, 54(1), 57-59 (1991-01-01)
The pharmacokinetics of a new Soviet-made immunostimulant kemantane, a derivative of adamantine, was studied by gas-liquid chromatography in patients with bronchial pathology. It was found that in the blood of the patients kemantane was not practically detected due to a
[The immunomodulator kemantan in the treatment of patients with exacerbated chronic obstructive bronchitis].
E M Rekalova
Likars'ka sprava, (4)(4), 73-76 (1992-04-01)
An expeditious preparation of E-2-amino-5-hydroxyadamantane and its Z-isomer.
Jaroskova L, et al.
Tetrahedron Letters, 47(46), 8063-8067 (2006)
Biocatalytic production of 5-hydroxy-2-adamantanone by P450cam coupled with NADH regeneration.
Furuya T, et al.
Journal of Molecular Catalysis. B, Enzymatic, 94, 111-118 (2013)
S S Boĭko et al.
Eksperimental'naia i klinicheskaia farmakologiia, 57(6), 48-50 (1994-11-01)
The pharmacokinetics of the new immunostimulant kemantane, adamantane derivative, used in two species of animals (rats and rabbits) and man was studied. There were significant differences in the pharmacokinetics of kemantane and its active metabolite--adamantane-1,4-diol between the species.
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.