모든 사진(1)
About This Item
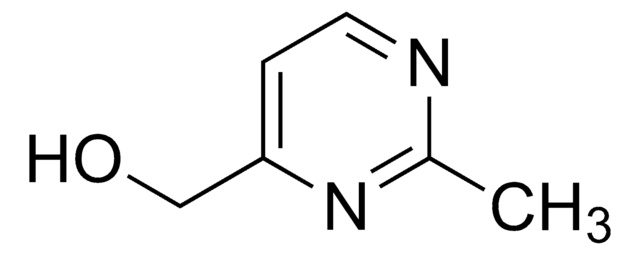
실험식(Hill 표기법):
C14H28N2O4S
CAS Number:
Molecular Weight:
320.45
Beilstein:
9559826
MDL number:
UNSPSC 코드:
12352108
eCl@ss:
32110502
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
≥99.0% (HPLC)
mp
98-100 °C
응용 분야
peptide synthesis
SMILES string
CC(C)(C)OC(=O)NN(SC(C)(C)C)C(=O)OC(C)(C)C
InChI
1S/C14H28N2O4S/c1-12(2,3)19-10(17)15-16(21-14(7,8)9)11(18)20-13(4,5)6/h1-9H3,(H,15,17)
InChI key
MOWYOPQOADUFLA-UHFFFAOYSA-N
기타 정보
Stable reagent for the preparation of asymmetric S-tert-butyl disulfide derivatives (e.g. for the introduction of S-tert-butylthio protection into cysteine peptides) in organic solvents[1]
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
E Wünsch et al.
Hoppe-Seyler's Zeitschrift fur physiologische Chemie, 363(12), 1461-1464 (1982-12-01)
The sterically hindered tert-butyl thiol reacts smoothly with azodicarboxylic acid derivatives only upon addition of catalytic amounts of sodium alcoholate, yielding crystalline and analytically well characterized 1-(tert-butylthio)-1,2-hydrazinedicarboxylic acid derivatives. These sulfur-activated reagents were found to be stable on storage and
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.