추천 제품
vapor pressure
14.46 psi ( 55 °C)
3.67 psi ( 20 °C)
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.423 (lit.)
bp
54-55 °C (lit.)
density
0.927 g/mL at 25 °C (lit.)
작용기
ether
저장 온도
2-8°C
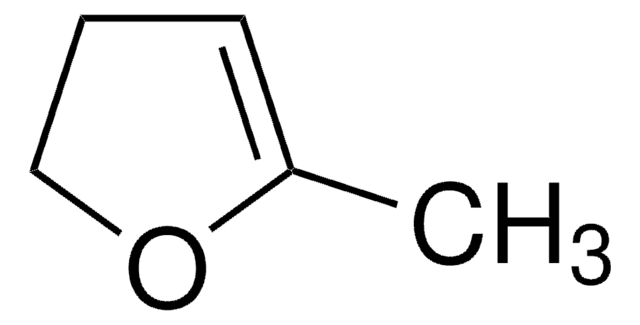
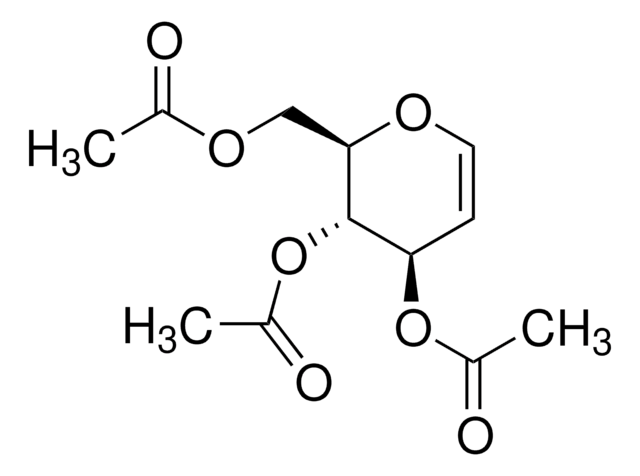
SMILES string
C1CC=CO1
InChI
1S/C4H6O/c1-2-4-5-3-1/h1,3H,2,4H2
InChI key
JKTCBAGSMQIFNL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The enantioselective Heck arylation of 2,3-dihydrofuran with aryl iodides was studied.
애플리케이션
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-11.2 °F - closed cup
Flash Point (°C)
-24 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
Claudia G Cobo-Angel et al.
Scientific reports, 9(1), 14025-14025 (2019-10-03)
Group B Streptococcus (GBS), is a leading cause of neonatal death and an emerging pathogen in adults. Additionally, GBS is a bovine pathogen causing intramammary infections. The likelihood of GBS interspecies transmission is largely unknown. We explored the potential transmission
The Journal of Organic Chemistry, 59, 4535-4535 (1994)
Lun-Zhi Dai et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(23), 7011-7018 (2008-07-08)
The gold(I)-catalyzed cycloisomerization of epoxy alkynes in the presence of a nucleophile is an efficient protocol to provide ketal skeletons with high stereoselectivity. An intramolecular reaction of propargylic/homopropargylic alcohols with oxirane to produce ketal/spiroketals in moderate yields under mild conditions
Raúl Pérez-Ruiz et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 5(1), 51-55 (2006-01-06)
The bicyclic oxetanes and resulting from photocycloaddition of aromatic aldehydes to 2,3-dihydrofuran, were efficiently cleaved by means of electron-transfer reduction, photoinduced by the electronically excited reductants 1-methoxynaphthalene (MN) and 2,7-dimethoxynaphthalene (DMN) in acetonitrile. The fluorescence quenching rates of DMN/MN by
Peizhong Xie et al.
Chemistry, an Asian journal, 7(7), 1533-1537 (2012-04-19)
A new domino reaction for Nazarov reagents: An efficient approach was developed for the construction of highly functionalized conjugated 2,3-dihydrofuran skeletons. Nazarov reagents were used for the first time in a phosphine-catalyzed domino reaction and successfully used to construct five-membered
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.