추천 제품
분석
97%
양식
solid
mp
272-276 °C (lit.)
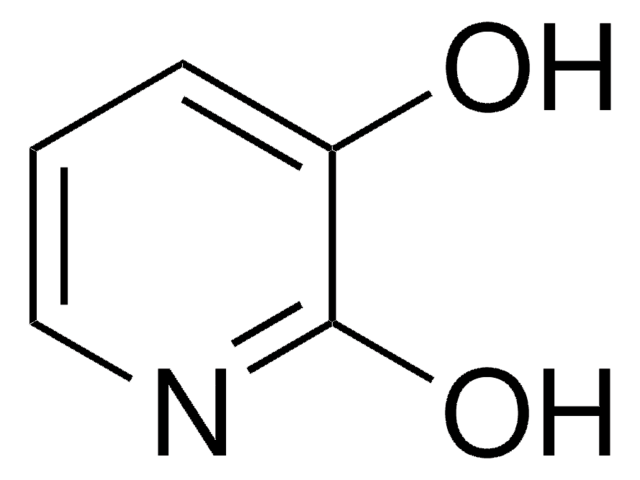
SMILES string
Oc1ccnc(O)c1
InChI
1S/C5H5NO2/c7-4-1-2-6-5(8)3-4/h1-3H,(H2,6,7,8)
InChI key
ZEZJPIDPVXJEME-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
M T Cocco et al.
European journal of medicinal chemistry, 35(5), 545-552 (2000-07-12)
4-hydroxy-2-pyridone derivatives 2 were prepared by reaction of 3-amino-3-dialkylaminopropenoates with bis(2,4, 6-trichlorophenyl)malonate. These compounds were further reacted with a set of aldehydes to give bis(pyridyl)methanes 3 and 4. The newly synthesized compounds 2, 3 and 4 were evaluated in vitro
F N Naguib et al.
Biochemical pharmacology, 38(9), 1471-1480 (1989-05-01)
One hundred and five nucleobase analogues were screened as inhibitors of dihydrouracil dehydrogenase (DHUDase, EC 1.3.1.2) from mouse liver. 5-Benzyloxybenzyluracil, 1-deazauracil (2,6-pyridinediol), 3-deazauracil (2,4-pyridinediol), 5-benzyluracil, 5-nitrobarbituric acid and 5,6-dioxyuracil (alloxan) were identified as potent inhibitors of this activity, with apparent
F P LaCreta et al.
Cancer research, 49(10), 2567-2573 (1989-05-15)
The breakdown of 5-fluoro-2'-deoxyuridine (FdUrd) to 5-fluorouracil (FUra) is catalyzed by the pyrimidine nucleoside phosphorylases, uridine phosphorylase and thymidine phosphorylase. The effects of nucleoside phosphorylase inhibitors on FdUrd and FUra elimination by the isolated perfused rat liver were investigated. The
M Kaneko et al.
Nucleic acids symposium series, (12)(12), 13-16 (1983-01-01)
New method for a synthesis of diazaphenoxathiin skeleton from 3-deazauracil derivatives is reported. It became possible to convert 3-deazauridine to 3-deazacytidine via an excellent intermediate "diazaphenoxathiin sulfoxide derivative".
K T Lin et al.
Therapeutic drug monitoring, 5(4), 491-496 (1983-01-01)
A rapid and simple procedure for liquid chromatographic analysis of plasma 3-deazauridine (3-DU), an antineoplastic agent, was developed. The plasma was extracted with methanolic silver acetate to remove interfering ultraviolet-absorbing materials and the 3-DU partially purified on a small anion
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.