AOAC Method 2011.11: UHPLC/MS/MS Analysis of Vitamin D2 and Vitamin D3 on Titan C18
CONDITIONS
column
Titan C18, 10 cm x 2.1 mm I.D., 1.9 μm particles (577124-U)
mobile phase
[A] 0.1% formic in methanol:water (20:80); [B] 0.1% formic acid in methanol
gradient
60 to 90% B in 0.4 min; to 100% B in 0.3 min; held at 100% B for 7.8 min; to 60% B in 0.1 min; held at 60% B for 1.5 min
flow rate
0.25 mL/min for 5.55 min; to 0.50 mL/min in 0.1 min; held at 0.5 mL/min for 3.84 min; to 0.25 mL/min in 0.1 min; held at 0.25 mL/min for 0.7 min
pressure
5859 psi (404 bar)
column temp.
25 °C
detector
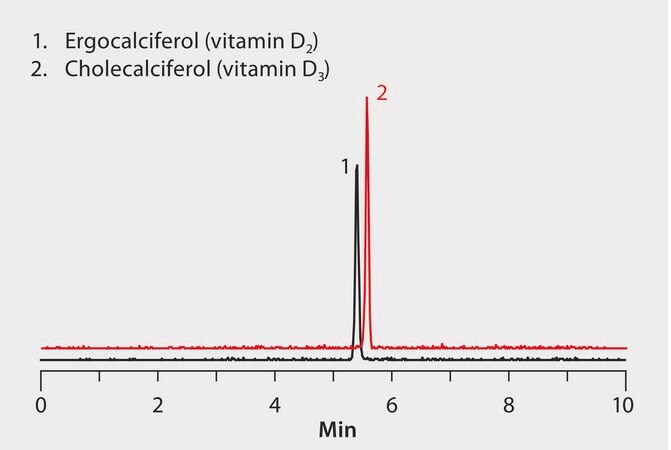
MS, APCI(+), MRM 397/125 (ergocalciferol, D2), MRM 385/259 (cholecalciferol, D3)
injection
5 μL
sample
1 IU/mL in methanol
詳細
アナリシスノート
AOAC method 2011.11 describes the procedure the analysis of vitamin D in infant formula and adult/pediatric nutritional formula using UHPLC/MS/MS. Two forms of vitamin D are recognized, vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol. Separation of vitamin D2 and D3 following the AOAC method is shown here using a Titan C18 UHPLC column.
法的情報
null