すべての画像(2)
About This Item
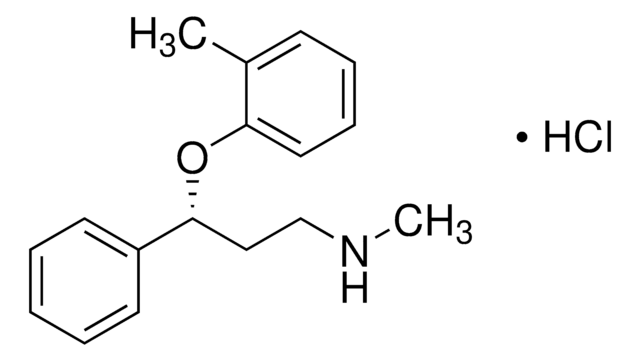
実験式(ヒル表記法):
C17H21NO · HCl
CAS番号:
分子量:
291.82
MDL番号:
UNSPSCコード:
12352200
PubChem Substance ID:
NACRES:
NA.77
おすすめの製品
形状
solid
オーガナイザー
Eli Lilly
保管温度
2-8°C
SMILES記法
CNCC[C@@H](OC1=CC=CC=C1C)C2=CC=CC=C2.[H]Cl
InChI
1S/C17H21NO.ClH/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15;/h3-11,17-18H,12-13H2,1-2H3;1H/t17-;/m1./s1
InChI Key
LUCXVPAZUDVVBT-UNTBIKODSA-N
遺伝子情報
human ... SLC6A2(6530)
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
(R)-Tomoxetine hydrochloride has been used as a noradrenaline reuptake inhibitor:
- to study the role of L-threo-3,4-dihydroxyphenylserine (L-DOPS) in the pathogenesis of Alzheimer′s disease in mice
- to study its effects on set shifting in rats
- to study its effects on rat brain as a result of its long-term use
生物化学的/生理学的作用
(R)-Tomoxetine hydrochloride is an efficient inhibitor of presynaptic norepinephrine transporters. It also positively regulates the release of acetylcholine in the prefrontal cortex (PFC). (R)-Tomoxetine hydrochloride binds to the serotonin (5-HT) transporter. It is involved in blocking the cortical N-methyl-D-aspartate (NMDA) receptors.. (R)-Tomoxetine hydrochloride exhibits therapeutic effects against attention-deficit/hyperactivity disorder (ADHD) and comorbid oppositional defiant disorder (ODD).
ノルエピネフリン取り込みのブロッカ-です。
特徴および利点
This compound is featured on the Biogenic Amine Transporters page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Eli Lilly. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
T7947-50MG:
T7947-BULK:
T7947-100MG:
T7947-VAR:
T7947-25MG:
T7947-5MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Fatma Gür et al.
Biochemical and biophysical research communications, 534, 927-932 (2020-11-05)
Attention Deficit Hyperactivity Disorder (ADHD) is the most common psychiatric disorder reported particularly in children. Long-term use of antipsychotic drugs used in the treatment of ADHD has been shown to exert toxic effects on the brain. However, not enough research
Grazia Dell'Agnello et al.
European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 19(11), 822-834 (2009-09-01)
The primary aim of this study was to assess the efficacy of atomoxetine in improving ADHD and ODD symptoms in paediatric patients with ADHD and comorbid oppositional defiant disorder (ODD), non-responders to previous psychological intervention with parent support. This was
Romano Arcieri et al.
Journal of child and adolescent psychopharmacology, 22(6), 423-431 (2013-01-31)
The purpose of this study was to assess the cardiovascular effects of drugs used for attention-deficit/hyperactivity disorder (ADHD) in children and adolescents treated in community care centers in Italy. This study was an open, prospective, observational study of youth with
Himanshu P Upadhyaya et al.
Psychopharmacology, 226(2), 189-200 (2013-02-12)
Treatment of attention-deficit/hyperactivity disorder (ADHD) has for many years relied on psychostimulants, particularly various formulations of amphetamines and methylphenidate. These are central nervous system stimulants and are scheduled because of their abuse potential. Atomoxetine (atomoxetine hydrochloride; Strattera®) was approved in
Todd M Durell et al.
Journal of clinical psychopharmacology, 33(1), 45-54 (2013-01-02)
Attention-deficit/hyperactivity disorder (ADHD) is associated with significant impairment in multiple functional domains. This trial evaluated efficacy in ADHD symptoms and functional outcomes in young adults treated with atomoxetine. Young adults (18-30 years old) with ADHD were randomized to 12 weeks
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)