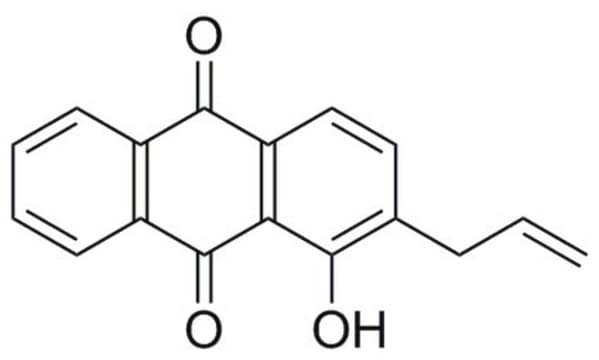
おすすめの製品
由来生物
Penicillium islandicum
品質水準
アッセイ
≥98% (HPLC)
保管条件
protect from light
溶解性
DMSO: soluble
acetone: soluble
ethyl acetate: soluble
保管温度
−20°C
InChI
1S/C30H18O10/c1-9-3-11-19(13(31)5-9)29(39)23-17(35)7-15(33)21(25(23)27(11)37)22-16(34)8-18(36)24-26(22)28(38)12-4-10(2)6-14(32)20(12)30(24)40/h3-8,31-36H,1-2H3
InChI Key
MQSXZQXHIJMNAF-UHFFFAOYSA-N
生物化学的/生理学的作用
Skyrin is a non-peptidic anthraquinone, mycotoxin with in vitro cytotoxic activity. It suppresses the growth of HeLa, Vero, K562, Raji, Wish and Calu-1 cell lines. Skyrin treatment induces DNA fragmentation and other morphological changes leading to apoptosis in Human HL-60 promyelotic leukemia cells. Skyrin was found to serve as an anti-diabetic agent by selectively binding to the glucagon receptor acting as its antagonist. Regular binding of glucagon to its receptor on hepathocyte plasma membrane activates adenylate cyclase indirectly. As a result, the cAMP produced activates protein kinase A with a consequent increase in both glycogen breakdown and gluconeogenesis leading to glucose output. Skyrin was shown to block this signal transduction sequence, such that the interaction of glucagons with its receptor does not result in an increase in cAMP production. Skyrin was also found to efficiently scavenge free radical species as •OH, •R and of singlet oxygen (1O2). Based on Skyrin selective toxicity towards insect cell line Sf9, it may be useful as an agent for pest control.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML0459-BULK:
SML0459-1MG-PW:
SML0459-1MG:
SML0459-VAR:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Jie Ma et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 37(16), 2408-2412 (2012-12-14)
To study the chemical constituents from Hypericum perforatum. Compouds were isolated by chromatographic techniques. Their structures were identified by spectral methods. The inhibitory activity of recombinant human PTP1B was evaluated. Nine compounds were elucidated as D-Mannitol (1), 1,2-benzenedicarboxylic acid bis(1-methylpropyl)
S Krivobok et al.
Mutation research, 279(1), 1-8 (1992-05-01)
Unsubstituted anthraquinone, 4 substituted anthraquinones (emodin, danthron, physcion, a new compound M-108-C) and 3 dimers (skyrin, rugulosin, rugulin) were tested using the Ames/Salmonella assay (strains TA98, TA100, TA1537 and TA102). Danthron and emodin were found to be mutagenic for TA1537
K Kawai et al.
Toxicology letters, 20(2), 155-160 (1984-02-01)
The anthraquinone mycotoxins emodin and skyrin were examined for the inhibitory effects on murine leukemia L1210 culture cells, oxidative phosphorylation of rat liver mitochondria, and Na+, K+-activated ATPase activity of rat brain microsomes to find the differences between their modes
K X Huang et al.
Current genetics, 28(6), 580-584 (1995-11-01)
Wild-type strains of Penicillium islandicum and Penicillium frequentans, which produce anthraquinone and related compounds, were transformed to benomyl and hygromycin B resistance. Plasmids pSV50 and pBT6, with benomyl-resistant beta-tublin genes, and plasmids pAN7-1 and pDH25, with a bacterial hygromycin phosphotransferase
Progress in the chemistry of organic natural products. The chemistry of mycotoxins.
S Bräse et al.
Progress in the chemistry of organic natural products, 97, v-xv (2013-06-21)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)