おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
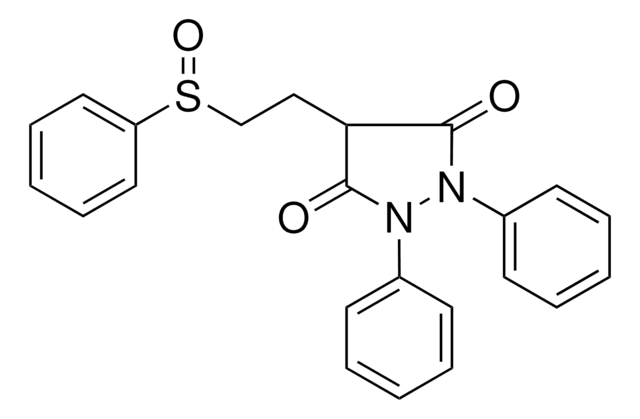
sulfinpyrazone
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
O=C1C(CCS(=O)c2ccccc2)C(=O)N(N1c3ccccc3)c4ccccc4
InChI
1S/C23H20N2O3S/c26-22-21(16-17-29(28)20-14-8-3-9-15-20)23(27)25(19-12-6-2-7-13-19)24(22)18-10-4-1-5-11-18/h1-15,21H,16-17H2
InChI Key
MBGGBVCUIVRRBF-UHFFFAOYSA-N
遺伝子情報
human ... SLC22A12(116085)
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Sulfinpyrazone for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
Choose from one of the most recent versions:
試験成績書(COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact カスタマーサポート
E H Margulies et al.
Drugs, 20(3), 179-197 (1980-09-01)
Sulfinpyrazone1 has long been recognised as a potent uricosuric agent, but has more recently been studied extensively as a platelet inhibitor and antithrombotic agent. It is active in man following oral administration and has been reported to be effective in
Sulfinpyrazone: relationship between dose, kinetics, plasma concentrations and biological effects.
M R Buchanan
Thrombosis research. Supplement, 4, 89-92 (1983-01-01)
Uricosuric drugs, with special reference to probenecid and sulfinpyrazone.
A B Gutman
Advances in pharmacology, 4, 91-142 (1966-01-01)
Andrew C Kotze et al.
Antimicrobial agents and chemotherapy, 58(12), 7475-7483 (2014-10-08)
We used an enzyme induction approach to study the role of detoxification enzymes in the interaction of the anthelmintic compound naphthalophos with Haemonchus contortus larvae. Larvae were treated with the barbiturate phenobarbital, which is known to induce the activity of
Ashley M Laughney et al.
Science translational medicine, 6(261), 261ra152-261ra152 (2014-11-08)
Eribulin mesylate was developed as a potent microtubule-targeting cytotoxic agent to treat taxane-resistant cancers, but recent clinical trials have shown that it eventually fails in many patient subpopulations for unclear reasons. To investigate its resistance mechanisms, we developed a fluorescent
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)