おすすめの製品
形状
powder
包装
pkg of 1 × 5 mg (860660P-5mg)
メーカー/製品名
Avanti Research™ - A Croda Brand 860660P
脂質タイプ
sphingolipids
輸送温度
dry ice
保管温度
−20°C
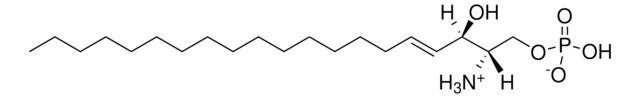
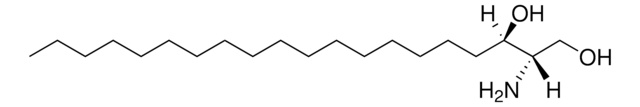
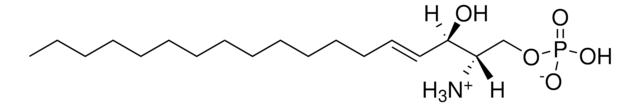
SMILES記法
OC[C@@](N)([H])[C@]([H])(O)/C=C/CCCCCCCCCCCCCCC
InChI
1S/C20H41NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20(23)19(21)18-22/h16-17,19-20,22-23H,2-15,18,21H2,1H3/b17-16+/t19-,20+/m0/s1
InChI Key
HTJSZHKGNMXZJN-YIVRLKKSSA-N
詳細
Sphingosine (d20:1), also known as icosasphingosine, is a synthetic sphingosine with 20 carbon long chain bases (LCB). It is directly produced by the sphingolipid de novo synthesis pathway and not by hydrolysis of complex sphingolipids. In higher animals, it is one of the most abundant sphingoid bases of gangliosides.
アプリケーション
Sphingosine (d20:1) has been used as a reference substance in liquid chromatography/tandem mass spectrometry analysis to quantitatively analyze sphingolipids with C20- long chain bases in human central nervous tissue.
生物化学的/生理学的作用
Sphingosine (d20:1) or C20-sphingosine acts as an intermediate in the synthesis of ceramides, which are vital components of mammalian epidermis. It may be involved in regulation of epidermal differentiation. C20 long chain bases-containing sphingolipids might exhibit detrimental effect on protein homeostasis and neural functions.
包装
5 mL Amber Glass Screw Cap Vial (860660P-5mg)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
860660P-5MG:
860660P-VAR:
860660P-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway.
Alfred H Merrill
The Journal of biological chemistry, 277(29), 25843-25846 (2002-05-16)
P W Wertz et al.
Biochimica et biophysica acta, 1002(2), 213-217 (1989-04-03)
Sphingosines and phytosphingosines serve as intermediates in the synthesis of ceramides and glucosylceramides, which are prominent components of mammalian epidermis. In the present study, we have investigated the possibility that free sphingoid bases also may be present in epidermal tissue.
Lihong Zhao et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(42), 12962-12967 (2015-10-07)
Sphingolipids typically have an 18-carbon (C18) sphingoid long chain base (LCB) backbone. Although sphingolipids with LCBs of other chain lengths have been identified, the functional significance of these low-abundance sphingolipids is unknown. The LCB chain length is determined by serine
Rajkumar Vutukuri et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 34(3), 3932-3942 (2020-01-17)
Sphingosine 1-phosphate (S1P) signaling influences numerous cell biological mechanisms such as differentiation, proliferation, survival, migration, and angiogenesis. Intriguingly, our current knowledge is based solely on the role of S1P with an 18-carbon long-chain base length, S1P d18:1. Depending on the
Thomas Kolter
ISRN biochemistry, 2012, 506160-506160 (2012-01-01)
Gangliosides are sialic acid-containing glycosphingolipids. They occur especially on the cellular surfaces of neuronal cells, where they form a complex pattern, but are also found in many other cell types. The paper provides a general overview on their structures, occurrence
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)