930652
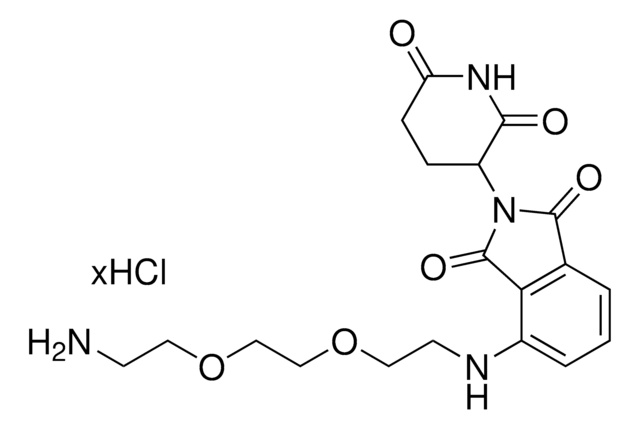
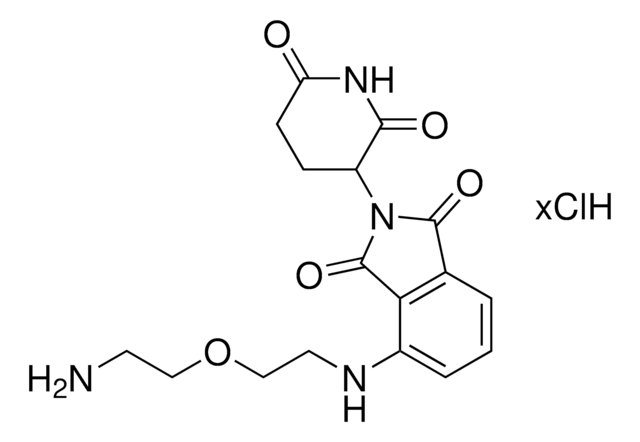
Pomalidomide-C2-NH2 hydrochloride
≥95%
別名:
4-((2-aminoethyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride, 4-[(2-Aminoethyl)amino]-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione, E3 Ligase Ligand 17
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C15H16N4O4 · xHCl
CAS番号:
分子量:
316.31 (free base basis)
MDL番号:
UNSPSCコード:
12352101
NACRES:
NA.21
おすすめの製品
ligand
pomalidomide
品質水準
アッセイ
≥95%
形状
powder
官能基
amine
保管温度
2-8°C
SMILES記法
O=C1NC(C(CC1)N2C(C3=C(C2=O)C(NCCN)=CC=C3)=O)=O.Cl
アプリケーション
Pomalidomide-C2-NH2 Hydrochloride is a functionalized cereblon ligand for development of pomalidomide-based PROTACs. Allows rapid conjugation with carboxyl linkers due to the presence of an amine group via peptide coupling reactions. It is also active for linker attachment via reductive amination, and a basic building block for development of a protein degrader library.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
その他情報
Targeted Protein Degradation by Small Molecules
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
法的情報
PROTAC® is a registered trademark of Arvinas Operations, Inc., and is used under license.
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
930652-VAR:
930652-100MG:
930652-BULK:
930652-500MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Jingwei Shao et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany), 8(20), e2102555-e2102555 (2021-08-17)
DNA-binding proteins, including transcription factors (TFs), play essential roles in various cellular processes and pathogenesis of diseases, deeming to be potential therapeutic targets. However, these proteins are generally considered undruggable as they lack an enzymatic catalytic site or a ligand-binding
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
Philipp M Cromm et al.
Cell chemical biology, 24(9), 1181-1190 (2017-06-27)
Traditional pharmaceutical drug discovery is almost exclusively focused on directly controlling protein activity to cure diseases. Modulators of protein activity, especially inhibitors, are developed and applied at high concentration to achieve maximal effects. Thereby, reduced bioavailability and off-target effects can
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)