おすすめの製品
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
アプリケーション
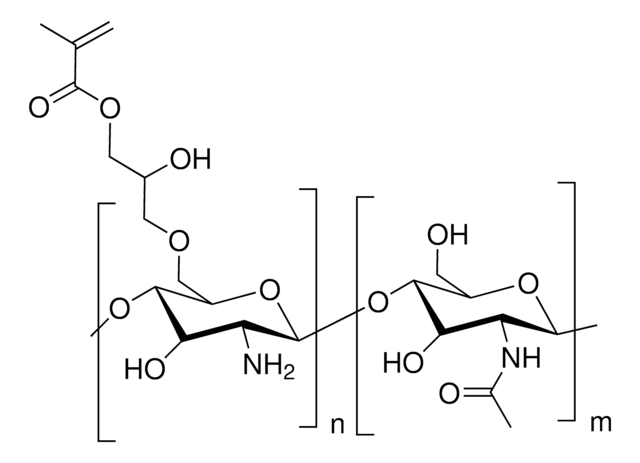
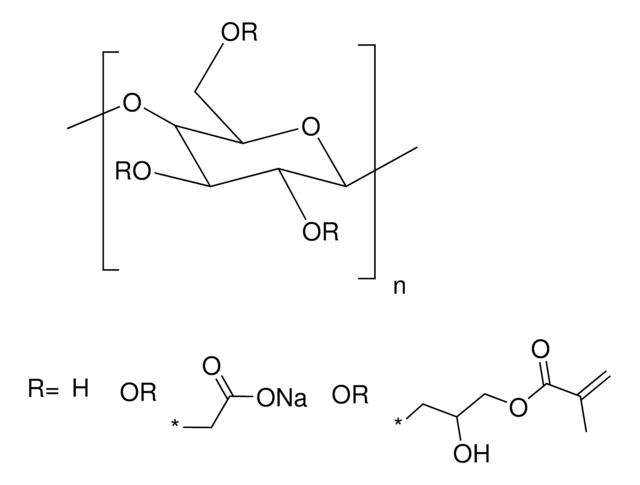
Alginate is an anionic polysaccharide that is widely used in pharmaceutical and biomedical applications due to its non-animal origin, low toxicity, biocompatibility, and biodegradability. Alginate hydrogels are commonly used to fabricate tissue engineering scaffolds, bioinks for 3D bioprinting, and nanocarriers for drug & gene delivery. While alginate is commonly crosslinked into a hydrogel via ionic-crosslinking with divalent cations (e.g., Ca2+), these gels feature limited long-term stability due to exchange reactions and migration of divalent cations from the alginate matrix. To prevent matrix degradation, alginate can be functionalized with reactive groups that can be chemically crosslinked, such as methacrylates. Methacrylate-functionalized alginate can be used to prepare hydrogels by thermal or photochemical crosslinking of the terminal methacrylates. Properties of the resulting hydrogel (e.g., stiffness, swelling ratio, rate of degradation) can be tuned by alginate molecular weight, degree of methacrylate functionalization, and crosslink density.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
912387-VAR:
912387-BULK:
912387-1G:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Photocrosslinkable polysaccharides for in situ hydrogel formation
Smeds K A and Grinstaff M W
Journal of Biomedical Materials Research Part A, 54 (1), 115-112 (2001)
Siddhesh N Pawar et al.
Biomaterials, 33(11), 3279-3305 (2012-01-28)
Alginates have become an extremely important family of polysaccharides because of their utility in preparing hydrogels at mild pH and temperature conditions, suitable for sensitive biomolecules like proteins and nucleic acids, and even for living cells such as islets of
Oju Jeon et al.
Biomaterials, 30(14), 2724-2734 (2009-02-10)
Photocrosslinked and biodegradable alginate hydrogels were engineered for biomedical applications. Photocrosslinkable alginate macromers were prepared by reacting sodium alginate and 2-aminoethyl methacrylate in the presence of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride and N-hydroxysuccinimide. Methacrylated alginates were photocrosslinked using ultraviolet light with 0.05% photoinitiator.
Andrew D Rouillard et al.
Tissue engineering. Part C, Methods, 17(2), 173-179 (2010-08-14)
Methods for seeding high-viability (>85%) three-dimensional (3D) alginate-chondrocyte hydrogel scaffolds are presented that employ photocrosslinking of methacrylate-modified alginate with the photoinitiator VA-086. Comparison with results from several other photoinitiators, including Irgacure 2959, highlights the role of solvent, ultraviolet exposure, and
Jia Jia et al.
Acta biomaterialia, 10(10), 4323-4331 (2014-07-08)
Recent advances in three-dimensional (3-D) printing offer an excellent opportunity to address critical challenges faced by current tissue engineering approaches. Alginate hydrogels have been used extensively as bioinks for 3-D bioprinting. However, most previous research has focused on native alginates
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)