おすすめの製品
アッセイ
98%
形状
solid
mp
123-126 °C (lit.)
溶解性
95% ethanol: soluble 50 mg/mL, clear, light yellow
SMILES記法
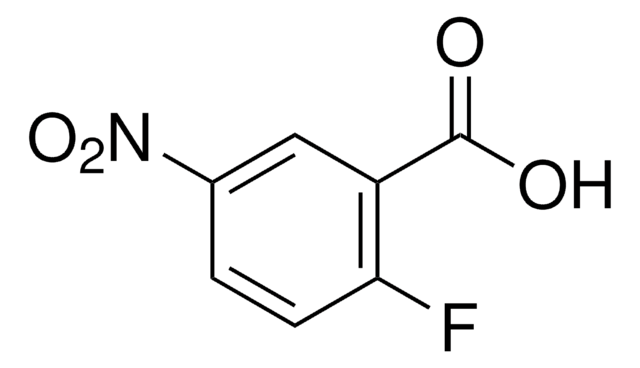
OC(=O)c1ccc(F)c(c1)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-5-2-1-4(7(10)11)3-6(5)9(12)13/h1-3H,(H,10,11)
InChI Key
BOJWTAQWPVBIPG-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
4-Fluoro-3-nitrobenzoic acid was used:
- as starting reagent in the preparation of novel benzimidazoles having antimycobacterial activity
- in preparation of series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure
- in preparation of bis(heterocyclic) skeletal precursors for the Pictet-Spengler reaction
- in solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
329045-25G:
329045-VAR:
329045-5G:
329045-1KG:
329045-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Yeong Keng Yoon et al.
European journal of medicinal chemistry, 93, 614-624 (2014-07-06)
A total of 51 novel benzimidazoles were synthesized by a 4-step reaction starting from basic compound 4-fluoro-3-nitrobenzoic acid under relatively mild reaction conditions. The structure of the novel benzimidazoles was confirmed by mass spectra as well as (1)H NMR spectroscopic
Yeong Keng Yoon et al.
Bioorganic chemistry, 49, 33-39 (2013-07-28)
Two series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure were synthesized by a four-step reaction pathway starting from 4-fluoro-3-nitrobenzoic acid as the basic compound. The structure of the novel benzimidazoles was characterized and confirmed by
J C Wijkmans et al.
Molecular diversity, 3(2), 117-120 (1997-01-01)
4-Fluoro-3-nitrobenzoic acid attached to a solid support was shown to react under mild conditions with a wide range of functionalized phenols to yield, after cleavage, the corresponding biaryl ethers in excellent purity. In a similar fashion, biaryl thioethers could be
Gérard Klein et al.
Journal of combinatorial chemistry, 4(4), 345-351 (2002-07-09)
An efficient method for the solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones from resin-bound amino acids is described. N-acylation of the primary amine of a resin-bound amino acid with 4-fluoro-3-nitrobenzoic acid, followed by displacement of the fluoro group and reduction of the
Wen-Bing Yeh et al.
Combinatorial chemistry & high throughput screening, 7(3), 251-255 (2004-05-12)
Liquid phasel synthesis of biheterocyclic benzimidazoles by controlled microwave irradiation was investigated. Polymer immobilized o-phenylenediamines was synthesized under microwave irradiation. The resulting PEG bound diamines was N-acylated with 4-fluoro-3-nitrobenzoic acid selectively in primary aromatic amino moiety. Nucleophilic aromatic substitution of
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)