SMB00113
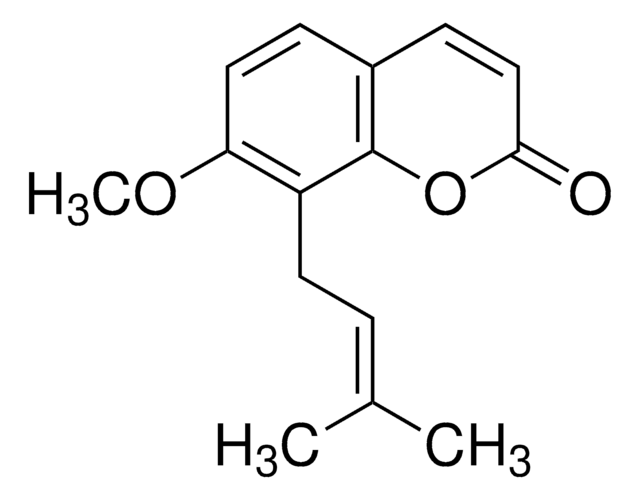
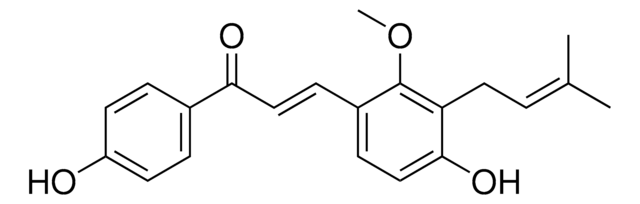
6-Geranyl-7-hydroxycoumarin
≥95% (LC/MS-ELSD)
Synonym(s):
6-(3,7-Dimethyl-2,6-octadienyl)-7-hydroxycoumarin, Ostruthin, Ostruthine
About This Item
Recommended Products
Assay
≥95% (LC/MS-ELSD)
form
solid
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
SMILES string
C\C(C)=C\CC\C(C)=C\Cc1cc2C=CC(=O)Oc2cc1O
InChI
1S/C19H22O3/c1-13(2)5-4-6-14(3)7-8-15-11-16-9-10-19(21)22-18(16)12-17(15)20/h5,7,9-12,20H,4,6,8H2,1-3H3/b14-7+
InChI key
INBMTJJPUABOQJ-VGOFMYFVSA-N
General description
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
SMB00113-1MG:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service