Q4951
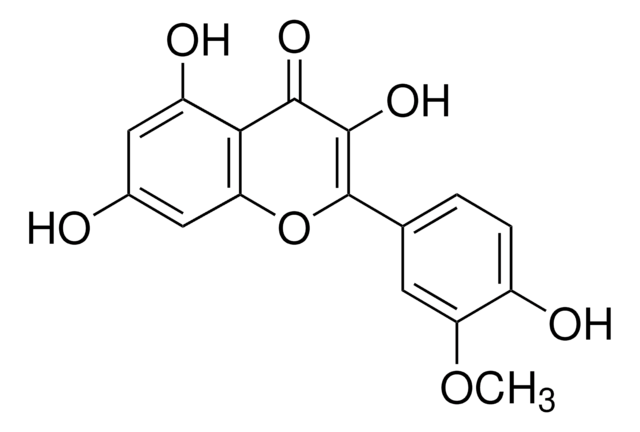
Quercetin
≥95% (HPLC), solid, anticancer agent
Synonym(s):
2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, 3,3′,4′,5,6-Pentahydroxyflavone
About This Item
Recommended Products
product name
Quercetin, ≥95% (HPLC), solid
biological source
synthetic (organic)
Assay
≥95% (HPLC)
form
solid
mp
316.5 °C
solubility
water: practically insoluble
storage temp.
room temp
SMILES string
OC1=CC(O)=C2C(OC(C3=CC=C(O)C(O)=C3)=C(O)C2=O)=C1
InChI
1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H
InChI key
REFJWTPEDVJJIY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Quercetin has been used as an antioxidant which reversed the immunosuppressive effects of high glucose and hyperglycemic sera in type 2 diabetic patients.
- It has been used as a detoxifying phytochemical in Apis mellifera.
- It has been used as a positive control in DPPH (2,2- diphenyl-1-picryhydrazyl) radical scavenging assay. It has also been used for the preparation of calibration curve to determine total flavonoid content.
Biochem/physiol Actions
Features and Benefits
Preparation Note
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
Q4951-BULK:
Q4951-VAR:
Q4951-10G:
Q4951-100G:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for Kinase Phosphatase Biology
Discover Bioactive Small Molecules for Lipid Signaling Research
Protocols
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service