CLLS1060
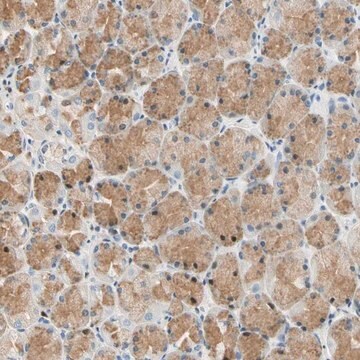
MCF10A CELLS CDC25B (-/-)
human female maMilliporeary glands (Source Disease: Fibrocystic Disease)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
41106514
eCl@ss:
32190102
NACRES:
NA.81
Prodotti consigliati
product name
MCF10A CELLS CDC25B (-/-),
Origine biologica
human female mammary glands (Source Disease: Fibrocystic Disease)
N° accesso OMIM
Temperatura di conservazione
−196°C
Informazioni sul gene
human ... CDC25B(994)
Descrizione generale
MCF10A CELLS CDC25B (-/-) are pre-neoplastic mammary epithelial cells from a human adult female with a ZFN knock out modification.
This cell line corresponds to ATCC Catalog No. CRL-10317.
Applicazioni
Cell freezing medium-DMSO 1X, Cat. No. C6164
Media renewal changes two to three times per week.
Rapidly thaw vial by gentle agitation in 37°C water bath (~2 minutes), keeping vial cap out of the water. Decontaminate with 70% ethanol, add 9 mL culture media and centrifuge 125 x g (5-7 minutes). Resuspend in complete culture media and incubate at 37°
The base medium for this cell line is Dulbecco′s Modified Eagle′s Medium/Ham′s Nutrient Mixture F-12, Cat. No. 51448C. To make the complete growth medium, add the following components to the base medium: horse serum, Cat No. H1270, to a final concentration (v/v) of 5%; cholera toxin, Cat No. C8052, to a final concentration of 1 ng/mL; human insulin, Cat No. I9278, to a final concentration of 10 ug/mL; epidermal growth factor, Cat No. E9644, to a final concentration of 10 ng/mL; and hydrocortisone, Cat No. H6909, to a final concentration of 0.5 ug/mL.
Caratteristiche e vantaggi
These MCF10A cells are adherent with a doubling time of approx. 20 hours
Zinc finger nuclease (ZFN) knockout on chromosome 20p13
Qualità
Tested for Mycoplasma, sterility, post-freeze viability, short terminal repeat (STR) analysis for cell line identification, cytochrome oxidase I (COI) analysis for cell line species confirmation.
Esclusione di responsabilità
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.