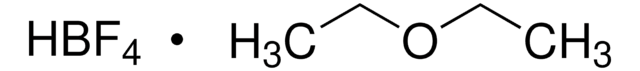207934
Tetrafluoroboric acid solution
48 wt. % in H2O
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HBF4
Numero CAS:
Peso molecolare:
87.81
Numero MDL:
Codice UNSPSC:
12352106
ID PubChem:
NACRES:
NA.21
Concentrazione:
46.0-52.0% in NaOH (titration)
48 wt. % in H2O
48 wt. % in H2O
Prodotti consigliati
Densità del vapore
3 (vs air)
Livello qualitativo
Tensione di vapore
5 mmHg ( 20 °C)
Stato
liquid
Concentrazione
46.0-52.0% in NaOH (titration)
48 wt. % in H2O
Densità
1.4 g/mL at 25 °C
Stringa SMILE
F.FB(F)F
InChI
1S/BF3.FH/c2-1(3)4;/h;1H
LEMQFBIYMVUIIG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Tetrafluoroboric acid is used as a catalyst for the protection and deprotection reactions of various carbohydrates. It participates in the synthesis of 4-sulfonic acid phenyl diazonium tetrafluoroborate, which was required for the preparation of sulfonated graphene (SG).
Applicazioni
Tetrafluoroboric acid solution may be used as a catalyst for the hydration of aromatic haloalkynes to α-halomethyl ketones in the absence of metal catalysts. It may also be used the epoxidized soybean oil (ESBO) ring opening step of fatty acids preparation.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Metal-free hydration of aromatic haloalkynes to a-halomethyl ketones.
Ye M, et al.
Tetrahedron Letters, 57(45), 4983-4986 (2016)
Self-assembling sulfonated graphene/polyaniline nanocomposite paper for high performance supercapacitor.
Fan T, et al.
Synthetic Metals, 199, 79-86 (2015)
Polyols and polyurethanes prepared from epoxidized soybean oil ring-opened by polyhydroxy fatty acids with varying OH numbers.
Chen R, et al.
Journal of Applied Polymer Science, 132(1) (2015)
Tetrafluoroboric acid, an efficient catalyst in carbohydrate protection and deprotection reactions.
Albert R, et al.
Carbohydrate Research, 137, 282-290 (1985)
Mario Latronico et al.
Inorganic chemistry, 50(8), 3539-3558 (2011-03-19)
The protonation of the phosphinito-bridged Pt(I) complex [(PHCy(2))Pt(μ-PCy(2)){κ(2)P,O-μ-P(O)Cy(2)}Pt(PHCy(2))](Pt-Pt) (1) by aqueous HBF(4) or hydrofluoric acid leads selectively to the hydrido-bridged solvento species syn-[(PHCy(2))(H(2)O)Pt(μ-PCy(2))(μ-H)Pt(PHCy(2)){κP-P(OH)Cy(2)}](Y)(2)(Pt-Pt) ([2-H(2)O]Y(2)) {Y = BF(4), F(HF)(n)} when an excess of acid was used. On standing in halogenated solvents
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.