D134651
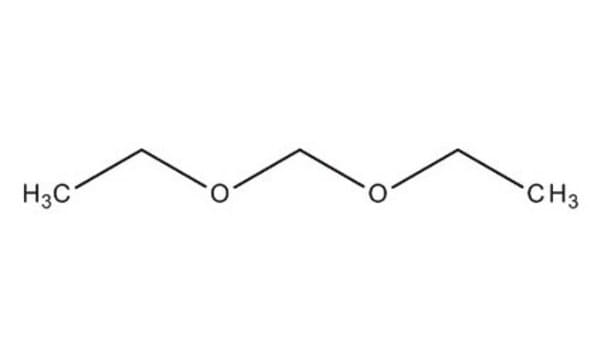
Dimethoxymethane
ReagentPlus®, 99%
Sinonimo/i:
Formaldehyde dimethyl acetal, Methylal
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH2(OCH3)2
Numero CAS:
Peso molecolare:
76.09
Beilstein:
1697025
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Densità del vapore
2.6 (vs air)
Livello qualitativo
Tensione di vapore
6.38 psi ( 20 °C)
Nome Commerciale
ReagentPlus®
Saggio
99%
Forma fisica
liquid
Temp. autoaccensione
459 °F
Limite di esplosione
17.6 %
Indice di rifrazione
n20/D 1.354 (lit.)
P. eboll.
41-42 °C (lit.)
Punto di fusione
−105 °C (lit.)
Densità
0.86 g/mL at 25 °C (lit.)
Stringa SMILE
COCOC
InChI
1S/C3H8O2/c1-4-3-5-2/h3H2,1-2H3
NKDDWNXOKDWJAK-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Dimethoxymethane (DMM, methylal) is a biodegradable dimethyl acetal. It can be synthesized by acid catalyzed condensation of formaldehyde with methanol. It is amphiphilic in nature with low viscosity, surface tension and boiling point. It is a flammable, highly volatile solvent with good dissolving power. DMM is considered as a potential alternative fuel and fuel additive due to its high oxygen content and its ability to enhance the combustion characteristics of diesel and petrol. Its thermal diffusivity has been determined by photoacoustic method. Analysis of the molecular structure of DMM by electron diffraction technique shows that it has C2 symmetry with a gauche-gauche conformation.
Applicazioni
Dimethoxymethane (Formaldehyde dimethyl acetal) may be used in the synthesis of methoxymethyl (MOM) ethers. It may also be used as an external cross-linker to form microporous polymers.
Note legali
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 2
Rischi supp
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
-0.4 °F - closed cup
Punto d’infiammabilità (°C)
-18 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
The molecular structure of dimethoxymethane.
Astrup EE.
Acta Chemica Scandinavica, 27(9), 3271-3276 (1973)
A photoacoustic study on Dimethoxymethane.
Bama GK and Ramachandran K.
AIP Conference Proceedings, 1004, 191-191 (2008)
A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker.
Li B, et al.
Macromolecules, 44(8), 2410-2414 (2011)
An efficient protocol for the preparation of MOM ethers and their deprotection using zirconium (IV) chloride.
Sharma GVM, et al.
Tetrahedron Letters, 45(50), 9229-9232 (2004)
Jenn-Huei Lii et al.
Carbohydrate research, 340(5), 853-862 (2005-03-23)
The rotational barrier for a methyl group at the end of an anomeric system is sometimes lower than we might have anticipated. Thus, in the trans-trans conformation of dimethoxymethane, the barrier to methyl rotation is calculated (B3LYP/6-311++G(2d,2p)) to be 2.22
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.