W249311
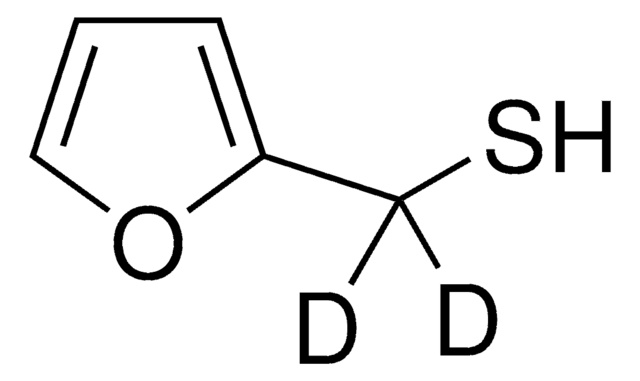
Furfuryl mercaptan
natural, 98%, FG
Sinonimo/i:
2-Furanmethanethiol, 2-Furfurylthiol, 2-Furylmethanethiol, Furfuryl mercaptan
About This Item
Prodotti consigliati
Grado
FG
Fragrance grade
Halal
Kosher
natural
agenzia
follows IFRA guidelines
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
Saggio
98%
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Indice di rifrazione
n20/D 1.531 (lit.)
P. eboll.
155 °C (lit.)
Densità
1.132 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Categoria alternativa più verde
Organolettico
coffee; meaty; roasted; sulfurous
Stringa SMILE
SCc1ccco1
InChI
1S/C5H6OS/c7-4-5-2-1-3-6-5/h1-3,7H,4H2
ZFFTZDQKIXPDAF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
113.0 °F - closed cup
Punto d’infiammabilità (°C)
45 °C - closed cup
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.