S550
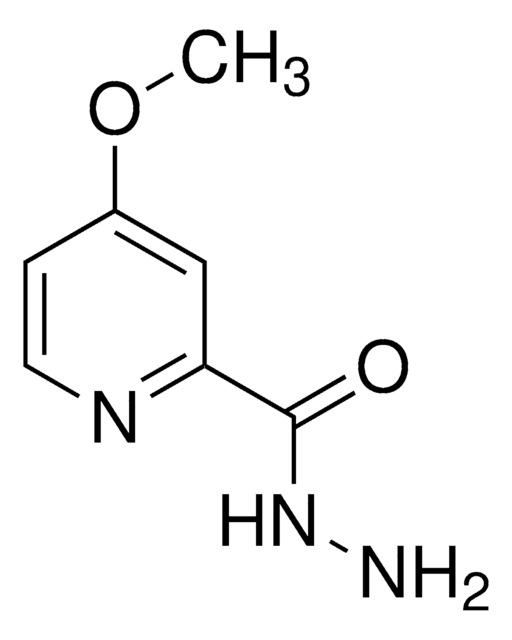
Salicyloyl hydrazide
98%
Sinonimo/i:
Salicyl hydrazide, Salicylhydrazide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
2-(HO)C6H4CONHNH2
Numero CAS:
Peso molecolare:
152.15
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
Prodotti consigliati
Saggio
98%
Punto di fusione
147-150 °C (lit.)
Stringa SMILE
NNC(=O)c1ccccc1O
InChI
1S/C7H8N2O2/c8-9-7(11)5-3-1-2-4-6(5)10/h1-4,10H,8H2,(H,9,11)
XSXYESVZDBAKKT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Paul S Francis
Luminescence : the journal of biological and chemical luminescence, 19(4), 205-208 (2004-08-03)
The chemiluminescence accompanying the oxidation of salicylic hydrazide (2-hydroxybenzoic acid hydrazide) with hypochlorite, hypobromite, N-chlorosuccinimide, N-bromosuccinimide or hydrogen peroxide with cobalt(II) matched the photoluminescence emission of salicylic acid. In a related reaction, the oxidation of a mixture of isoniazid and
Xuefei Cao et al.
Current cancer drug targets, 9(2), 189-201 (2009-03-12)
Previously, we described a series of salicylhydrazide compounds with potent anti-cancer activities against a panel of human cancer cell lines derived from different origins. Preclinical evaluation showing efficacy both in vitro and in vivo in human cancer models indicated that
Laith Q Al-Mawsawi et al.
Bioorganic & medicinal chemistry letters, 17(23), 6472-6475 (2007-10-24)
The previously discovered salicylhydrazide class of compounds displayed potent HIV-1 integrase (IN) inhibitory activity. The development of this class of compounds as antiretroviral agents was halted due to cytotoxicity in the nanomolar to sub-micromolar range. We identified a novel class
S A Thompson et al.
British journal of pharmacology, 142(1), 97-106 (2004-04-22)
1. A high-throughput assay utilizing the voltage/ion probe reader (VIPR) technology identified salicylidene salicylhydrazide (SCS) as being a potent selective inhibitor of alpha2beta1gamma1 GABA(A) receptors with a maximum inhibition of 56+/-5% and an IC(50) of 32 (23, 45) nm. 2.
Muhib Ahmed et al.
Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine, 32(4), 671-682 (2019-06-24)
Hydrazide ligand, (Z)-N'-(6-oxo-1,10-phenanthrolin-5(6H)-ylidene)isonicotinohydrazide, 1 forms from a 1:1 Schiff base condensation reaction between isoniazid (INH) and 1,10-phenanthroline-5,6-dione (phendione). Ag+ and Mn2+ complexes with 1:2 metal:ligand stoichiometry are prepared: [Ag(1)2]NO3, [Ag(1)2]BF4 and [Mn(1)2](NO3)2. Polymeric {[Ag(1)(NO3)]}n has 1:1 stoichiometry and forms upon
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.