633216
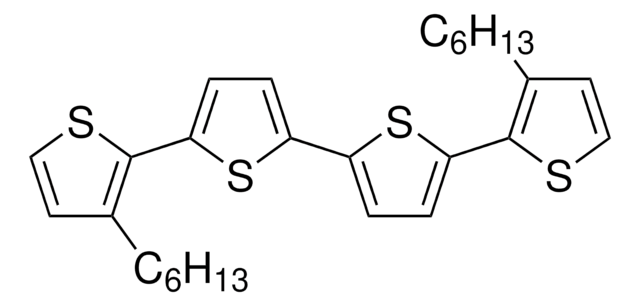
5,5′′′′′-Dihexyl-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′′:5′′′′,2′′′′′-sexithiophene
electron donor for OPV devices
Sinonimo/i:
α,ω-Dihexylsexithiophene, DH-6T
About This Item
Prodotti consigliati
Forma fisica
solid
Punto di fusione
280 °C (dec.) (lit.)
Solubilità
chlorobenzene: soluble (soluble)
chloroform: slightly soluble
methylene chloride: slightly soluble
Energia dell’orbitale
HOMO 5.2 eV
LUMO 2.9 eV
Prestazioni di un dispositivo OPV
ITO/DH6T/PC61BM/Al
ITO/PEDOT:PSS/DH6T/PC61BM/Al
ITO/PEDOT:PSS/DH6T:PC61BM (1:1)/Al
Caratteristiche del semiconduttore
P-type (mobility=0.13 cm2/V·s)
Stringa SMILE
CCCCCCc1ccc(s1)-c2ccc(s2)-c3ccc(s3)-c4ccc(s4)-c5ccc(s5)-c6ccc(CCCCCC)s6
InChI
1S/C36H38S6/c1-3-5-7-9-11-25-13-15-27(37-25)29-17-19-31(39-29)33-21-23-35(41-33)36-24-22-34(42-36)32-20-18-30(40-32)28-16-14-26(38-28)12-10-8-6-4-2/h13-24H,3-12H2,1-2H3
QCMASTUHHXPVGT-UHFFFAOYSA-N
Descrizione generale
Applicazioni
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Review the potential of self-assembled multilayer gate dielectric films fabricated from silane precursors for organic, inorganic, and transparent TFT and for TFT circuitry and OLED displays.
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Organic materials in optoelectronic devices like LEDs and solar cells are of significant academic and commercial interest.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![[(S)-(−)-1-(4-Nitrophenyl)-2-pyrrolidinemethyl]acrylate 97%](/deepweb/assets/sigmaaldrich/product/structures/194/557/0896d3d3-56d0-4b1e-9c08-1fdd246b5e86/640/0896d3d3-56d0-4b1e-9c08-1fdd246b5e86.png)